Published online Feb 6, 2020. doi: 10.12998/wjcc.v8.i3.587
Peer-review started: November 30, 2019
First decision: December 11, 2019
Revised: January 3, 2020
Accepted: January 8, 2020
Article in press: January 8, 2020
Published online: February 6, 2020
Pseudohypoparathyroidism type Ia (PHP Ia) is a rare hereditary syndrome, and patients with early PHP Ia are generally not diagnosed based on the presentation of cutaneous nodules as the main clinical feature. Here, we describe a Chinese boy with PHP Ia in whom the main clinical feature was cutaneous nodules, and the patient exhibited a novel GNAS mutation.
A 5-year-old boy presented with a 5-year history of cutaneous nodules scattered over his entire body. The patient had a short stature, round face, short neck, and slightly flattened nose; he also had multiple hard papules and cutaneous nodules scattered over his entire body. The patient had a significantly elevated parathyroid hormone level. His serum calcium level was reduced, while his serum phosphorus level was increased and his serum thyroid-stimulating hormone level was elevated. Skin biopsy showed osteoma cutis in subcutaneous tissue. Sanger sequencing revealed a frameshift mutation, c.399delT (p.Ser133Argfs*2) in exon 5 of the GNAS gene. The patient was diagnosed with PHP Ia and subclinical hypothyroidism. He was given 1,25-dihydroxyvitamin D, calcium carbonate, and synthetic L-thyroxine. After 3 months of treatment, the patient’s parathyroid hormone level decreased, and his serum calcium and serum phosphorus levels were normal. Moreover, his thyroid-stimulating hormone level decreased.
These findings can help dermatologists to diagnose PHP Ia in patients with cutaneous nodules as the main early clinical feature.
Core tip: Herein, we describe a Chinese boy with pseudohypoparathyroidism type Ia who presented with multiple cutaneous nodules at an early stage and exhibited a c.399delT mutation in the GNAS gene. This mutation was not found in public databases, including Exome Aggregation Consortium, the Genome Aggregation Database, ClinVar, and the Human Gene Mutation Database. Therefore, we concluded that the c.399delT mutation in exon 5 of the GNAS gene was a novel mutation associated with pseudohypoparathyroidism type Ia.
- Citation: Li YL, Han T, Hong F. Cutaneous nodules and a novel GNAS mutation in a Chinese boy with pseudohypoparathyroidism type Ia: A case report and review of literature. World J Clin Cases 2020; 8(3): 587-593
- URL: https://www.wjgnet.com/2307-8960/full/v8/i3/587.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i3.587
Pseudohypoparathyroidism (PHP) is a rare, sporadic or familial disorder characterized by an elevated plasma level of parathyroid hormone (PTH) caused by resistance to PTH action in target tissues[1,2]. PHP was first reported in 1942 by Albright et al[3] and classified into PHP type I (PHP I) and PHP type II (PHP II) according to the renal response to exogenous PTH administration. PHP I is divided into subtypes Ia, Ib, and Ic. Patients with PHP Ia display hypocalcemia, hyper-phosphatemia, PTH resistance, and variable developmental abnormalities [e.g., Albright’s hereditary osteodystrophy (AHO)]. PHP Ia is caused by inactivating mutations of the guanine nucleotide-binding protein, alpha-stimulating activity polypeptide (GNAS) gene, which encodes the alpha subunit of the stimulatory heterotrimeric G protein (Gsα)[4].
Patients with PHP Ia generally seek medical attention due to epileptic seizures, and both the misdiagnosis and missed diagnosis rates are extremely high. Here, we describe a patient with PHP Ia who presented with multiple cutaneous nodules as the main clinical feature; this patient did not experience epileptic seizures and exhibited a novel GNAS mutation.
A 5-year-old boy with a 5-year history of cutaneous nodules was referred to the Children’s Hospital at Zhejiang University School of Medicine, Hangzhou, China.
Cutaneous nodules had been observed since the patient was 2 mo old. A hard papule and nodules were first found at the waist. Numerous similar lesions subsequently appeared on the head, trunk, and limbs; these gradually became progressively larger.
The patient had no significant medical history, psychiatric history, or history of substance misuse.
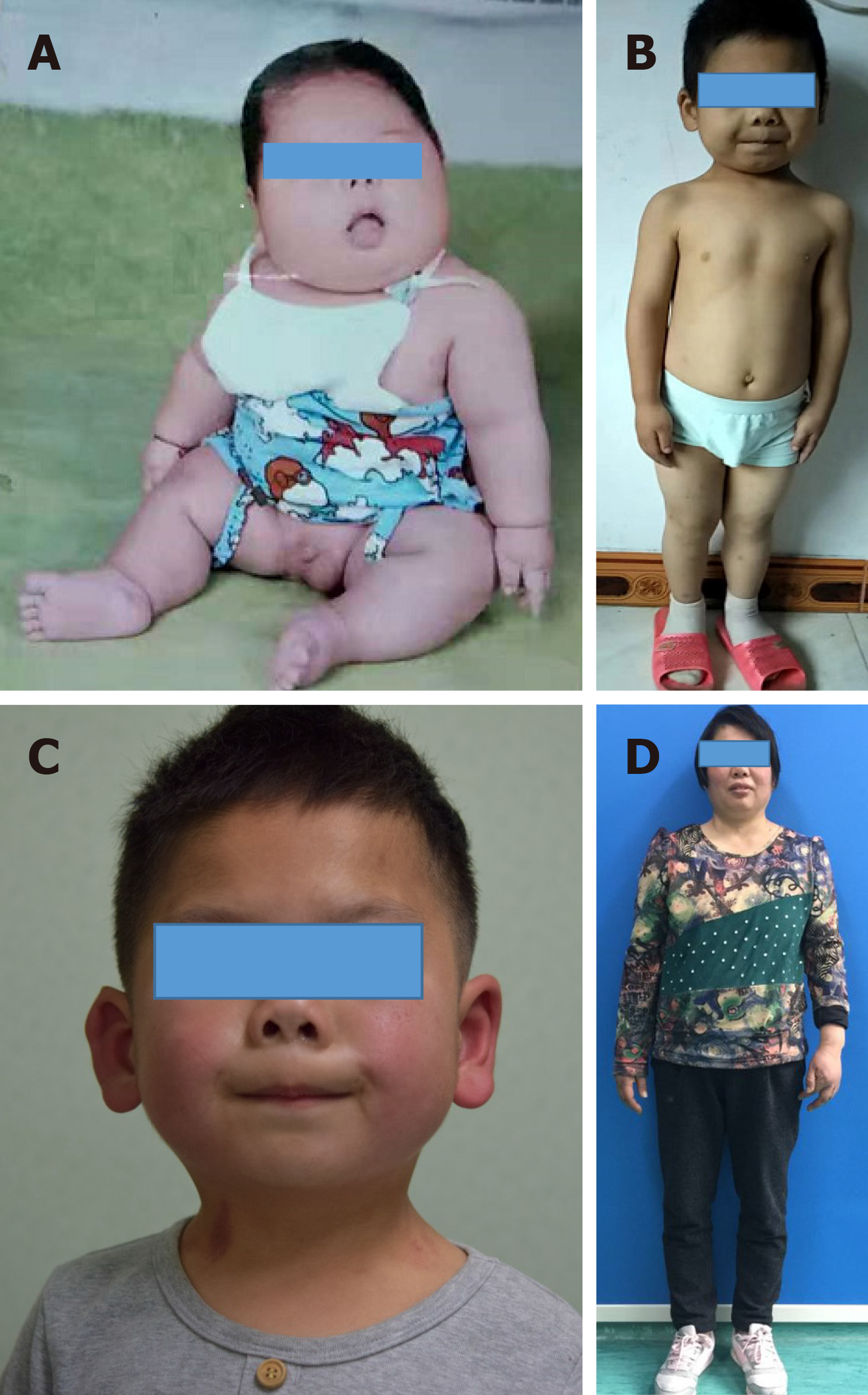
The patient was born at a gestational age of 34 wk with a birth weight of 2.6 kg and height of 46 cm. The patient’s weight was between the 6th and 10th percentiles from birth to the age of 3 mo. His weight then began to increase rapidly such that obesity was evident at the age of 4 mo (Figure 1A) and continued until the age of 9 mo (> 97th percentile). The patient’s height was between the 6th and 10th percentiles from birth to the time of this report. The patient could raise his head at the age of 6 mo, sit up at the age of 1 year, and walk at the age of 2 years. The patient’s mother had exhibited similar cutaneous nodules in the calf and trunk regions at the age of 26 years; she also had a short stature, round face, and was obese (Figure 1D). The patient’s older brother, father, aunt, and uncle did not display similar manifestations of the disease.
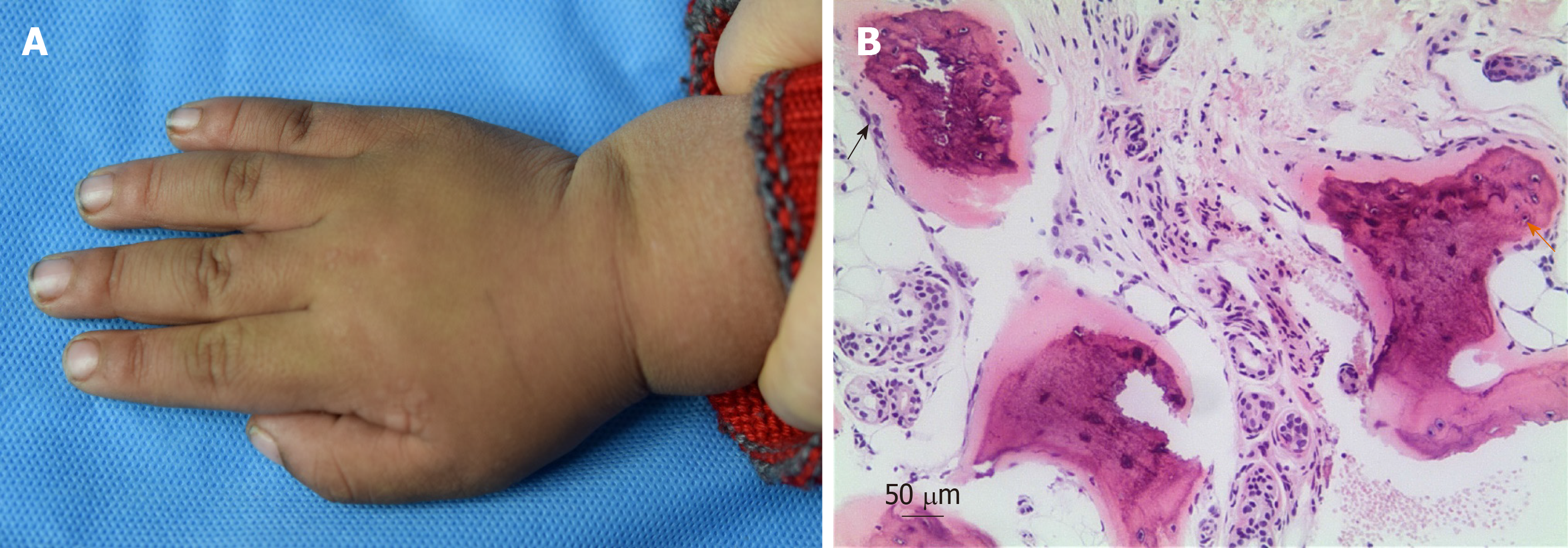
On physical examination, the child had a short stature, round face, short neck, and slightly flattened nose (Figure 1B and C). His height was 105 cm and his weight was 19.5 kg (10th and 75th percentiles, respectively). The patient was only able to express simple sentences; his score on the Wechsler Intelligence Scale for Children was 51, which suggested mental retardation. The patient had multiple hard papules and cutaneous nodules scattered over his entire body; these cutaneous nodules measured 1 mm to 2 cm in size (Figure 2A). He exhibited no erythema, blisters, tenderness, or swelling on the surfaces of cutaneous nodules.
Laboratory investigations showed normal findings in routine blood, urine, and stool tests, along with normal liver and renal function. The patient also had normal cortisol, adrenocorticotropic hormone, bone type alkaline phosphatase, and 25-hydroxyvitamin D levels. His serum calcium level was 2.03 mmol/L (normal range, 2.20-2.65 mmol/L) and his serum phosphorus level was 2.91 mmol/L (normal range, 1.29-2.26 mmol/L); his serum PTH level was significantly elevated (466.2 ng/L; normal range, 15-65 ng/L). The patient had an elevated thyroid-stimulating hormone (TSH) level of 12.34 mIU/L (normal range, 0.35-4.94 mIU/L) with a normal thyroxine level of 71.87 nmol/L (normal range, 62.68-150.80 nmol/L) and a normal triiodothyronine level of 2.01 nmol/L (normal range, 0.88-2.44 nmol/L). A skin biopsy from his right thigh showed heterotopic ossification within the subcutaneous tissue, indicative of osteoma cutis (Figure 2B). Sanger sequencing showed that a c.399delT heterozygous gene mutation (NM_000516.4) was present in exon 5 of GNAS in both the patient and his mother.
Brain computed tomography examination showed multiple calcium-like high-density calcifications in the patient’s scalp, but not within the brain. Electroencephalogram showed no seizure-like waves.
The proband was diagnosed with PHP Ia by the presence of hormone resistance, cutaneous ossification, obesity, and AHO phenotype, and maternal inheritance of the GNAS mutation. This proband had elevated TSH levels and normal T3 and T4 levels, so a diagnosis of subclinical hypothyroidism was made. Therefore, the final diagnosis of the patient was PHP Ia combined with subclinical hypothyroidism.
The patient was given 1,25-dihydroxyvitamin D at a dose of 0.25 μg once daily and calcium carbonate at a dose of 300 mg once daily; he was instructed to avoid dairy, soy, and other high-phosphorus food products. Because the patient’s subclinical hypothyroidism led to concerns regarding his neurologic development, he was also administered synthetic L-thyroxine at a dose of 12.5 μg once daily.
After 3 mo of treatment, the patient’s PTH level decreased to 184.10 ng/L (normal range, 15-65 ng/L) with serum calcium and phosphorus levels of 2.24 (normal range, 2.20-2.65 mmol/L) and 2.05 mmol/L (normal range, 1.29-2.26 mmol/L), respectively; his TSH level decreased to 8.12 mIU/L (normal range, 0.35-4.94 mIU/L).
PHP is a rare autosomal dominant inherited disorder that is associated with an exon mutation in the maternal allele of the GNAS gene. The GNAS gene is located on chromosome 20q13, which consists of 13 exons and 12 introns. The product of the GNAS gene is Gsα, which is an important component of the cAMP/protein kinase. PTH mainly couples with Gsα through the PTH receptor to form a complex that activates adenylate cyclase and promotes the generation of cAMP, thereby regulating the cell response. GNAS is a complex imprinted gene encoding Gsα that exhibit exclusively maternal or paternal expression[5]. Maternal allele of the GNAS gene is the only source of Gsα in the kidney (the paternal allele is normally silenced in this tissue). In PHP Ia, mutations in the maternal GNAS allele result in a marked reduction of Gsα levels, leading to failure to elicit an appropriate increment in urinary cAMP and phosphate excretion following exogenous PTH infusion and PTH resistance[6]. Because the target organs (i.e., the kidney and bone) do not respond to PTH, hypocalcemia feedback stimulates excessive parathyroid secretion of PTH.
The clinical manifestations of PHP Ia include signs of hypoparathyroidism, characterized by calcium and phosphorus metabolism disorders[7-9]. Some patients also demonstrate a typical AHO phenotype, including short stature, obesity, round face, short neck, short and coarse fingers/toes, and ectopic ossification. The molecular mechanisms of GNAS-related heterotopic ossification have not been fully elucidated. It might involve aberrant differentiation of mesenchymal progenitor cells in the dermis or subcutaneous fat. Researchers have reported that activation of Hedgehog signaling causes GNAS-related heterotopic ossification. In PHP Ia with mutations in GNAS, Hedgehog signaling is activated in progenitor cells, which leads to heterotopic ossification and inhibition of formation of adipocytes[10,11]. Heterotopic calcification or ossification is extremely common in patients with AHO, which mainly occurs in the subcutaneous tissue and brain parenchyma; the most common manifestation is calcification of the brain basal ganglia. Seizures can also be caused by brain parenchymal calcification and hypocalcemia. Thyroid hormone, growth hormone, and gonadotropin are mediated by Gsα; therefore, patients with a GNAS mutation may present with resistance to other hormones, including TSH, growth hormone, and gonadotropin-releasing hormone. Therefore, patients with PHP Ia may exhibit hypothyroidism and hypogonadism. The patient in this case had an elevated TSH level and normal triiodothyronine and thyroxine levels; thus, a diagnosis of subclinical hypothyroidism was made.
PHP Ia needs to be distinguished from PHP Ib, PTH Ic, and pseudo-PHP. PHP type Ib is a rare genomic disease that is typically sporadic; a minority of patients demonstrate autosomal dominant inheritance. PHP Ib is associated with abnormal epigenetic regulation of the GNAS gene due to the loss of GNAS promoter methylation; the GNAS gene coding sequence is unaltered. In addition to PTH resistance, the clinical manifestations of PHP Ib typically include resistance to many other hormones. PHP Ib is generally not accompanied by AHO characteristics, but some patients may exhibit short fingers and toes. Patients with PTH Ic exhibit AHO characteristics and resistance to multiple hormones, but have normal Gsα activity; thus far, the molecular and genetic mechanisms of PTH Ic remain unknown. Pseudo-PHP is a syndrome caused by a mutation of the paternal allele of the GNAS gene; affected patients exhibit AHO characteristics, as well as normal serum calcium and normal serum phosphorus, but lack PTH resistance.
There have been previous reports of patients with PHP Ia in whom the diagnosis was confirmed by genetic analysis. PHP Ia cases with GNAS mutations previously described are reviewed[12-33] and summarized in Table 1. For example, two Korean patients with PHP Ia were confirmed to have a nonsense mutation of c.94A>T (p.Lys32X) and a known frameshift mutation of c.344_345insT (p.V117RfsX23) in the GNAS gene[24]. Another study of patients with PHP Ia revealed two novel mutations: A c.569_570del mutation (p.Tyr190CysfsX19) and a splicing mutation (c.659+1G>A) in the GNAS gene[25]. A Turkish boy was reported to have PHP type Ia (GNAS gene mutation, IVS4+5G>C); his mother had pseudo-PHP[33]. Here, we describe a 5-year-old boy with PHP Ia who had the c.399delT (p.Ser133Argfs*2) mutation in the GNAS gene; his mother carried the same mutation. Notably, the C.399delT mutation of exon 5 in the GNAS gene is a frameshift mutation; this frameshift mutation causes AGT→AGG, resulting in an Arg to Ser amino acid variant. The second codon after this mutation site (c.399delT) becomes the stop codon (TGA), which induces early termination of translation and leads to a truncated protein associated with partial PTH resistance. We searched for this mutation in published online databases including Exome Aggregation Consortium, genome Aggregation Database, ClinVar database, and Human Gene Mutation Database; there was no information about the site in these databases. Therefore, we concluded that the c.399delT mutation in the GNAS gene is novel.
| Mutation | Protein change | Ref. |
| c.753C>G | p.Ser251Arg | Warner et al[12], 1997 |
| c.302_303delAG | p.Glu101Glyfs*3 | Yu et al[13], 1999 |
| c.305C>A | p.Ala102Glu | Ahrens et al[14], 2001 |
| c.347C>T | p.Pro116Leu | Ahrens et al[14], 2001 |
| c.348_349insT | p.Val117Cysfs*23 | de Sanctis et al[15], 2004 |
| c.839G>A | p.Arg280Lys | Linglart et al[16], 2006 |
| c.546delC | p.Ile182Metfs*3 | Gelfand et al[17], 2007 |
| c.191A>T | p.His64Leu | Long et al[18], 2007 |
| c.750C>G | p.Ser250Arg | Long et al[18], 2007 |
| c.637C>T | p.Gln213* | Balavoine et al[19], 2008 |
| c.728del | p.Thr243fs | Adegbite et al[20], 2008 |
| c.517G>A | p.Asp173Asn | Freson et al[21], 2008 |
| c.871delC | p.Leu291Serfs*44 | Sun et al[22], 2009 |
| c.93delG | p.Lys32Argfs*26 | Thiele et al[23], 2010 |
| c.94A>T | p.Lys32X | Park et al[24], 2010 |
| c.569_570del | p.Tyr190CysfsX19 | Jin et al[25], 2011 |
| c.659+1G>A | Splicing mutation | Jin et al[25], 2011 |
| c.695G>A | p.Arg232His | Ishikawa et al[26], 2011 |
| c.85C>T | p.Gln29* | Cho et al[27], 2013 |
| c.457C>G | p.Leu153Val | Cho et al[27], 2013 |
| c.91C>T | p.Gln31* | Fernandez-Rebollo et al[28], 2013 |
| c.330G>C | p.Met110Ile | Fernandez-Rebollo et al[28], 2013 |
| c.21dupT | p.Lys8c | Elli et al[29], 2013 |
| c.344_345insT | p.Val117Argfs*23 | Elli et al[29], 2013 |
| c.360delC | p.Asn121Thrfs*12 | Elli et al[29], 2013 |
| c.860_861delTG | p.Val287Aspfs*12 | Elli et al[29], 2013 |
| c.682C>T | p.Arg228Cys | Tam et al[30], 2014 |
| c.496C>T | p.Arg166Cys | Thiele et al[31], 2015 |
| c.568_571del | p.Asp190fs | Lemos et al[32], 2015 |
| IVS4+5G>C | Kırel et al[33], 2016 |
PHP Ia is a rare genetic disease with an extremely high rate of missed diagnosis. The findings in this report can help dermatologists to recognize and accurately diagnose PHP Ia in patients with cutaneous nodules as the main early clinical feature. If necessary, genetic testing should be conducted to confirm the diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Faias S S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Liu MY
| 1. | Mantovani G. Clinical review: Pseudohypoparathyroidism: diagnosis and treatment. J Clin Endocrinol Metab. 2011;96:3020-3030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Weinstein LS, Yu S, Warner DR, Liu J. Endocrine manifestations of stimulatory G protein alpha-subunit mutations and the role of genomic imprinting. Endocr Rev. 2001;22:675-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Albright F, Burnett CH, Smith PH. Pseudohypoparathyroidism: An example of ‘Seabright-Bantam syndrome. Endocrinology. 1942;30:922-932. [Cited in This Article: ] |
| 4. | Spiegel AM. Hormone resistance caused by mutations in G proteins and G protein-coupled receptors. J Pediatr Endocrinol Metab. 1999;12(Suppl 1):303-309. [Cited in This Article: ] |
| 5. | Hayward BE, Kamiya M, Strain L, Moran V, Campbell R, Hayashizaki Y, Bonthron DT. The human GNAS1 gene is imprinted and encodes distinct paternally and biallelically expressed G proteins. Proc Natl Acad Sci U S A. 1998;95:10038-10043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 192] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Psychou F, Nicolaidou P, Georgouli H, Maniati-Christidis M, Dacou-Voutetakis C. Pseudohypoparathyroidism type Ia and growth hormone deficiency. Growth hormone releasing hormone receptor defect? Hormones (Athens). 2002;1:47-50. [PubMed] [Cited in This Article: ] |
| 7. | Donghi V, Mora S, Zamproni I, Chiumello G, Weber G. Pseudohypoparathyroidism, an often delayed diagnosis: a case series. Cases J. 2009;2:6734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Breslau NA. Pseudohypoparathyroidism: current concepts. Am J Med Sci. 1989;298:130-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Al-Azem H, Khan AA. Hypoparathyroidism. Best Pract Res Clin Endocrinol Metab. 2012;26:517-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Lietman SA, Ding C, Cooke DW, Levine MA. Reduction in Gsalpha induces osteogenic differentiation in human mesenchymal stem cells. Clin Orthop Relat Res. 2005;434:231-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Bastepe M. GNAS mutations and heterotopic ossification. Bone. 2018;109:80-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Warner DR, Gejman PV, Collins RM, Weinstein LS. A novel mutation adjacent to the switch III domain of G(S alpha) in a patient with pseudohypoparathyroidism. Mol Endocrinol. 1997;11:1718-1727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Yu D, Yu S, Schuster V, Kruse K, Clericuzio CL, Weinstein LS. Identification of two novel deletion mutations within the Gs alpha gene (GNAS1) in Albright hereditary osteodystrophy. J Clin Endocrinol Metab. 1999;84:3254-3259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Ahrens W, Hiort O, Staedt P, Kirschner T, Marschke C, Kruse K. Analysis of the GNAS1 gene in Albright's hereditary osteodystrophy. J Clin Endocrinol Metab. 2001;86:4630-4634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | de Sanctis L, Vai S, Andreo MR, Romagnolo D, Silvestro L, de Sanctis C. Brachydactyly in 14 genetically characterized pseudohypoparathyroidism type Ia patients. J Clin Endocrinol Metab. 2004;89:1650-1655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Linglart A, Mahon MJ, Kerachian MA, Berlach DM, Hendy GN, Jüppner H, Bastepe M. Coding GNAS mutations leading to hormone resistance impair in vitro agonist- and cholera toxin-induced adenosine cyclic 3',5'-monophosphate formation mediated by human XLalphas. Endocrinology. 2006;147:2253-2262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Gelfand IM, Hub RS, Shore EM, Kaplan FS, Dimeglio LA. Progressive osseous heteroplasia-like heterotopic ossification in a male infant with pseudohypoparathyroidism type Ia: a case report. Bone. 2007;40:1425-1428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Long DN, McGuire S, Levine MA, Weinstein LS, Germain-Lee EL. Body mass index differences in pseudohypoparathyroidism type 1a versus pseudopseudohypoparathyroidism may implicate paternal imprinting of Galpha(s) in the development of human obesity. J Clin Endocrinol Metab. 2007;92:1073-1079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Balavoine AS, Ladsous M, Velayoudom FL, Vlaeminck V, Cardot-Bauters C, d'Herbomez M, Wemeau JL. Hypothyroidism in patients with pseudohypoparathyroidism type Ia: clinical evidence of resistance to TSH and TRH. Eur J Endocrinol. 2008;159:431-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Adegbite NS, Xu M, Kaplan FS, Shore EM, Pignolo RJ. Diagnostic and mutational spectrum of progressive osseous heteroplasia (POH) and other forms of GNAS-based heterotopic ossification. Am J Med Genet A. 2008;146A:1788-1796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Freson K, Izzi B, Labarque V, Van Helvoirt M, Thys C, Wittevrongel C, Bex M, Bouillon R, Godefroid N, Proesmans W, de Zegher F, Jaeken J, Van Geet C. GNAS defects identified by stimulatory G protein alpha-subunit signalling studies in platelets. J Clin Endocrinol Metab. 2008;93:4851-4859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Sun LH, Cui B, Zhao HY, Tao B, Wang WQ, Li XY, Ning G, Liu JM. Identification of a novel GNAS mutation for pseudohypoparathyroidism in a Chinese family. Endocrine. 2009;36:25-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Thiele S, Werner R, Ahrens W, Hübner A, Hinkel KG, Höppner W, Igl B, Hiort O. Selective deficiency of Gsalpha and the possible role of alternative gene products of GNAS in Albright hereditary osteodystrophy and pseudohypoparathyroidism type Ia. Exp Clin Endocrinol Diabetes. 2010;118:127-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Park CH, Park HD, Lee SY, Kim JW, Sohn YB, Park SW, Jin DK. Clinical, biochemical, and genetic analysis of korean patients with pseudohypoparathyroidism type Ia. Ann Clin Lab Sci. 2010;40:261-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Jin HY, Lee BH, Choi JH, Kim GH, Kim JK, Lee JH, Yu J, Yoo JH, Ko CW, Lim HH, Chung HR, Yoo HW. Clinical characterization and identification of two novel mutations of the GNAS gene in patients with pseudohypoparathyroidism and pseudopseudohypoparathyroidism. Clin Endocrinol (Oxf). 2011;75:207-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Ishikawa Y, Tajima T, Nakae J, Nagashima T, Satoh K, Okuhara K, Fujieda K. Two mutations of the Gsalpha gene in two Japanese patients with sporadic pseudohypoparathyroidism type Ia. J Hum Genet. 2001;46:426-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Cho SY, Yoon YA, Ki CS, Huh HJ, Yoo HW, Lee BH, Kim GH, Yoo JH, Kim SY, Kim SJ, Sohn YB, Park SW, Huh R, Chang MS, Lee J, Kwun Y, Maeng SH, Jin DK. Clinical characterization and molecular classification of 12 Korean patients with pseudohypoparathyroidism and pseudopseudohypoparathyroidism. Exp Clin Endocrinol Diabetes. 2013;121:539-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Fernandez-Rebollo E, García-Cuartero B, Garin I, Largo C, Martínez F, Garcia-Lacalle C, Castaño L, Bastepe M, Pérez de Nanclares G. Intragenic GNAS deletion involving exon A/B in pseudohypoparathyroidism type 1A resulting in an apparent loss of exon A/B methylation: potential for misdiagnosis of pseudohypoparathyroidism type 1B. J Clin Endocrinol Metab. 2010;95:765-771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Elli FM, deSanctis L, Ceoloni B, Barbieri AM, Bordogna P, Beck-Peccoz P, Spada A, Mantovani G. Pseudohypoparathyroidism type Ia and pseudo-pseudohypoparathyroidism: the growing spectrum of GNAS inactivating mutations. Hum Mutat. 2013;34:411-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Tam VH, Chen SP, Mak CM, Fung LM, Lee CY, Chan AY. A novel mutation in pseudohypoparathyroidism type 1a in a Chinese woman and her son with hypocalcaemia. Hong Kong Med J. 2014;20:258-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Thiele S, Werner R, Grötzinger J, Brix B, Staedt P, Struve D, Reiz B, Farida J, Hiort O. A positive genotype-phenotype correlation in a large cohort of patients with Pseudohypoparathyroidism Type Ia and Pseudo-pseudohypoparathyroidism and 33 newly identified mutations in the GNAS gene. Mol Genet Genomic Med. 2015;3:111-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Lemos MC, Thakker RV. GNAS mutations in Pseudohypoparathyroidism type 1a and related disorders. Hum Mutat. 2015;36:11-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 33. | Kırel B, Demiral M, Bozdağ Ö, Karaer K. A novel mutation in a case of pseudohypoparathyroidism type Ia. Turk J Pediatr. 2016;58:101-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |