Published online Jan 26, 2020. doi: 10.12998/wjcc.v8.i2.337
Peer-review started: November 10, 2019
First decision: November 19, 2019
Revised: November 25, 2019
Accepted: December 6, 2019
Article in press: December 6, 2019
Published online: January 26, 2020
Japanese encephalitis (JE) is a serious public health concern with a high mortality rate in many Asian countries. For many years, JE virus (JEV) was considered the major cause of viral encephalitis in Asia. Although most JE cases are asymptomatic, the case fatality rate approaches 30%, and approximately 30%–50% of survivors have long-term neurological sequelae. To the best of our knowledge, JEV infection has never been reported following liver transplantation.
We report a case of a woman who underwent liver transplantation for autoimmune liver disease but presented with fever and neurological symptoms 13 d after transplantation. Magnetic resonance imaging revealed JEV infection, and positive immunoglobulin M antibody to JEV in blood and cerebrospinal fluid confirmed JE. The patient was treated with antiviral agents, immune regulation, and organ function support. No neurological sequelae were present after 1 year of follow-up.
Imaging and lumbar puncture examination should be performed as soon as possible in patients with fever and central nervous system symptoms after liver transplantation, and the possibility of atypical infection should be considered, which is helpful for early diagnosis and improved prognosis.
Core tip: Japanese encephalitis is a serious public health concern with a high mortality rate in many Asian countries. We describe a rare case of a woman who underwent liver transplantation and was subsequently diagnosed with Japanese encephalitis. This case highlights the need for performing imaging and lumbar puncture examination as soon as possible in patients with fever and central nervous system symptoms after liver transplantation.
- Citation: Qi ZL, Sun LY, Bai J, Zhuang HZ, Duan ML. Japanese encephalitis following liver transplantation: A rare case report. World J Clin Cases 2020; 8(2): 337-342
- URL: https://www.wjgnet.com/2307-8960/full/v8/i2/337.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i2.337
Liver transplantation is a fundamental therapeutic solution to end-stage liver disease[1,2]. However, infection is a major complication that causes significant morbidity and mortality after transplantation[3]. Despite advances in surgical techniques, liver transplant recipients are at high risk of infection because of immunosuppression. Bacteria, fungi, viruses, and parasites can cause infection both before and after transplantation[4]. As such, early recognition, along with timely and accurate diagnosis and treatment, plays a key role in the prognosis of infected individuals.
Japanese encephalitis (JE) is a serious public health concern with a high mortality rate in many Asian countries[5]. For many years, JE virus (JEV) was considered the major cause of viral encephalitis in Asia, with approximately 67900 cases reported each year. Although most JE cases are asymptomatic, the case fatality rate approaches 30%, and approximately 30%–50% of survivors have long-term neurological sequelae[6]. However, there has been no previous report of JE after liver transplantation[7].
A 67-year-old woman with autoimmune hepatitis cirrhosis was admitted for liver transplantation. At 13 d post-surgery, the patient developed fever of 38.4 °C. The next day, she complained of fatigue, nausea, and vomiting after eating.
This patient presented with myalgia, arthritis and arthralgias, photosensitivity, oral ulcers, keratoconjunctivitis sicca, and positive antinuclear antibody test 11 years ago, and she was diagnosed with systemic lupus erythematosus (SLE) then. Her arthralgias were improving with treatment, but right upper quadrant abdominal pain was worsening. Abdominal ultrasound showed evidence of cirrhosis 5 years ago, and she started to have recurrent hematemesis and melena 1 year ago. She underwent gastroscopic esophageal varies ligation 1 year ago as well as surgical aortic valve replacement due to severe aortic valve stenosis. She was diagnosed with SLE related autoimmune hepatitis with decompensated liver cirrhosis.
Her admission diagnosis was SLE related autoimmune hepatitis with decompensated liver cirrhosis, the Model for End-stage Liver Disease score was 38[8]. An appropriate donation after circulatory death was available on the day of admission, and the patient underwent orthotopic liver transplantation. The surgery time was 6 h 25 min, with intraoperative blood loss of 500 mL and transfusion of 400 mL of red blood cells. The patient was removed from the ventilator upon transfer to the intensive care unit (ICU) after surgery. Following surgery, cefepime was administered to prevent infection, along with methylprednisolone and tacrolimus to prevent graft rejection. Epstein–Barr virus test and 1, 3-β-D-Glucan Assay were positive, and Enterococcus faecalis was cultured from intraoperative donor liver lavage fluid and postoperative drainage fluid. As a result, ganciclovir, vancomycin, and micafungin were successively added to the treatment regimen to prevent infection, and liver function improved. Doppler ultrasonography on postoperative day 4 indicated the transplanted liver was normal in shape and size, with an anterior to posterior diameter of about 8.4 cm in the right lobe, homogeneous parenchyma echo, non-dilated internal and external bile ducts, and normal liver blood flow. The patient was transferred to a general ward 4 d after the operation.
By the afternoon of postoperative day 13, the patient’s body temperature increased to 38.9 °C, and she showed lethargy and weakness. From postoperative days 15 to 19, the patient showed persistent fever, with a temperature up to 42 °C. She developed progressive consciousness disorder, which gradually developed into shallow coma with no response to pain stimulation, neck resistance (+), continuous limb and trunk tremors, and higher limb muscle tension. The patient was returned to the ICU on day 19 post-surgery. At this point, the patient had a Glasgow coma scale score of 6, indicative of deep coma with intermittent convulsions, and a temperature of 38.6 °C.
On day 13 post-surgery, routine blood examination showed the following results: white blood cell (WBC) count, 11.67 × 109/L; granulocyte percentage, 90.8%; hemoglobin, 87 g/L; and platelet count, 58 × 109/L. Blood gas results were: pH, 7.42; PCO2, 33 mmHg; PO2, 66 mmHg; and base excess (BE), −3.2. Biochemical test results were: aspartate aminotransferase, 17 U/h; alanine aminotransferase, 8 U/h; albumin, 36 g/L; total bilirubin, 13.93 μmol/L (3.4–17.1 μmol/L); creatinine, 62 μmol/L; lactic acid, 0.5 mmol/L; K+, 4.01 mmol/L; and Na+, 129 mmol/L.
On the day of return to the ICU, blood test results were: WBC count, 7.45 × 109/L; granulocyte percentage, 85.4%; hemoglobin, 74 g/L; and platelet count, 88 × 109/L. Blood gas results were: pH, 7.45; PCO2, 32 mmHg; PO2, 158 mmHg; and BE, −1.9. Biochemical test results were: Aspartate aminotransferase (AST), 14 U/h; alanine aminotransferase, 4 U/h; albumin, 36 g/L; total bilirubin, 12.06 μmol/L; creatinine, 87 μmol/L; lactic acid, 0.5 mmol/L; K+, 4.51 mmol/L; and Na+, 131 mmol/L.
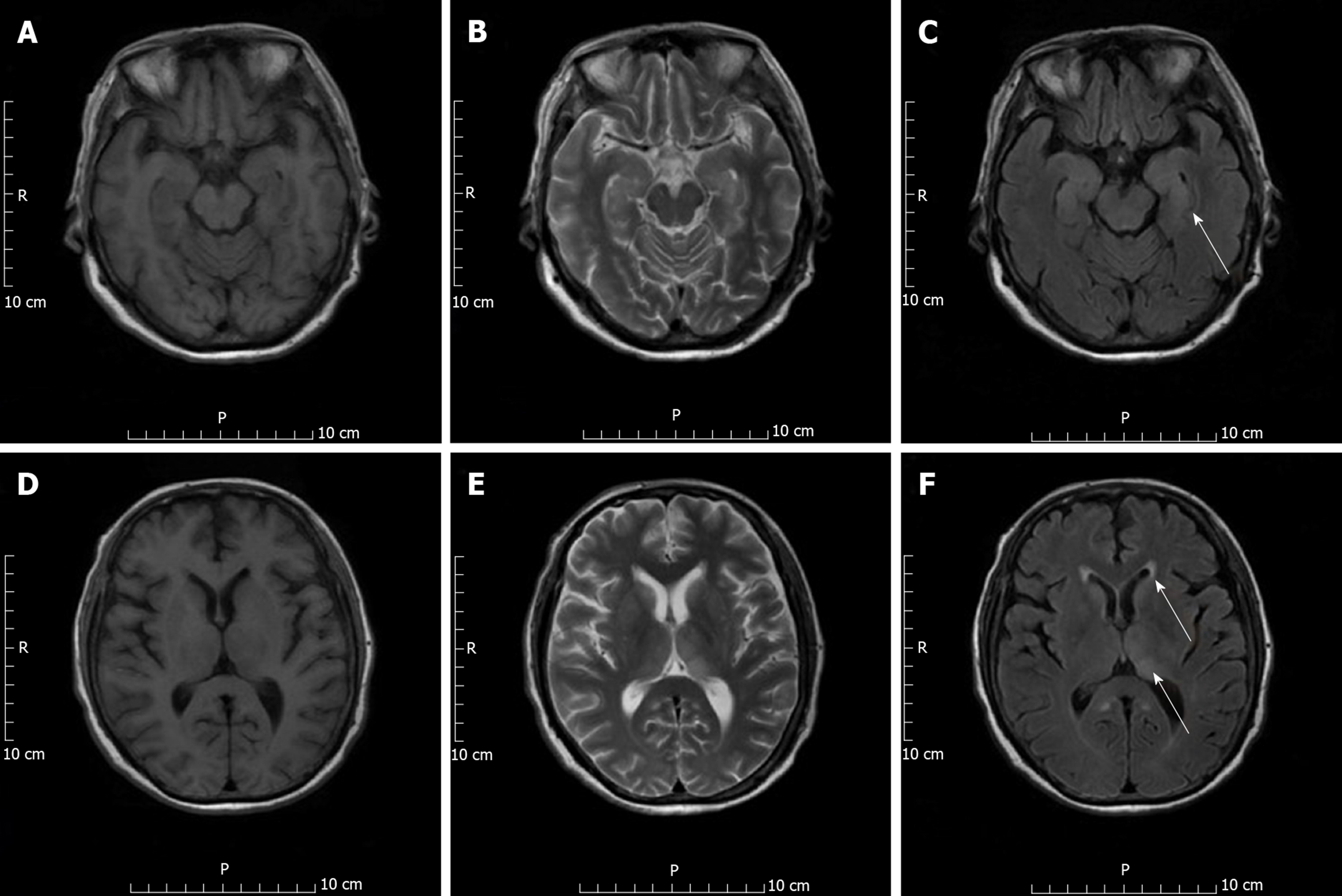
On day 13 post-surgery, chest computed tomography revealed lung bronchiectasis with infection and pleural effusion. Head computed tomography showed lacunar infarction. Head magnetic resonance imaging revealed abnormal signals in the bilateral thalamus and caudate nucleus, bilateral temporal sulcus gyrus, and parahippocampal gyrus, along with infectious lesions, leading us to suspect JEV infection (Figure 1).
On the day after return to the ICU, lumbar puncture revealed that cerebrospinal fluid pressure was 70 mmH2O; cerebrospinal fluid white blood cell count, 140 × 106/L (0–8 × 106/L); total albumin 244.67 mg/dL (15–45 mg/dL); and glucose, 4.96 mmol/L (2.24–3.92 mmol/L).
Serum and cerebrospinal fluid samples taken on day 20 were positive for JEV antibodies. And autoimmune encephalitis panel tests were negative in serum and cerebrospinal fluid, including N-methyl-D-aspartate receptor (NMDAR), leucine-rich glioma inactivated 1 (LGI1), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor 1 (AMPAR1), AMPAR2, gamma-aminobutyric acid B receptor (GABA-B receptor), contactin-associated protein-like 2 (Caspr2).
The final diagnosis of the present case was JE.
We applied acyclovir treatment and intravenous immunoglobulin. Midazolam was administered to control convulsions. On postoperative day 19, patient had a normal PO2 and PCO2 with Glasgow coma scale score of 6, and oropharyngeal airway and head of bed elevation were performed initially. In the following day, endotracheal intubation was undertaken due to deteriorated PCO2 to protect the patient’s airway. To prevent allograft rejection, methylprednisolone 25 mg q6 h intravenously (iv) (taper to 8 mg po once daily on day 7 post-surgery), tacrolimus 1.5 mg po q12 h, mycophenolate mofetil 750 mg po q12 h, and basiliximab 20 mg on day 0 and day 4 post-surgery were administered. On day 19 post-surgery, while patient’s mental status deteriorated, tacrolimus was discontinued, methylprednisolone doses were given 20 mg iv once daily, and mycophenolate mofetil was maintained at 750 mg q12 h po. Other treatments mainly included anti-infective agents and maintaining a stable internal environment. Anti-rejection regimen was changed to mycophenolate mofetil and methylprednisolone.
The patient’s mental status is improved; GCS score returned from 6 points back to 12 points; body temperature peak decreased; meningeal irritation disappeared; and limb muscle strength gradually recovered. On day 27 post-surgery, the patient was extubated and transferred to a general surgery ward on day 32. At 1 year post-surgery, the patient showed no signs of infection, had stable organ function, was conscious, and had no neurological sequelae.
Infectious complications are a major cause of morbidity and mortality following liver transplantation, despite recent advances in organ transplantation. Infection can be caused by bacteria, fungi, viruses, and even parasites. Infections occurring soon after transplantation are likely to be acquired during surgery from the donor organ or as a result of nosocomial pathogens. Opportunistic infections occur later and reflect the impact of immunosuppressive drugs. The risk of infection at any time after transplantation is determined by the status of immunosuppression, epidemiological exposure, vaccination, and chemoprophylaxis of the recipient[9].
The most common clinical manifestation of JE is acute encephalitis. After an incubation period of 5–15 d, initial symptoms are usually nonspecific and may include fever, diarrhea, and chills, followed by headache, vomiting, and general weakness. Changes in mental state, focal neurological dysfunction (including paresis, hemiplegia, quadriplegia, or cerebral palsy), or motor impairment can occur in the following days, with many patients falling into a coma and some requiring supplementary ventilation. In our case, on day 13 after liver transplantation, the patient developed fever, headache, general weakness, altered mental state, and hemiplegia, and then fell into a coma requiring mechanical ventilation. The clinical manifestations were consistent with JE. The patient had hyponatremia, and cerebrospinal fluid examination revealed elevated WBC count, elevated protein concentration, and a normal glucose concentration. Magnetic resonance imaging suggested abnormal signal lesions in the thalamus and caudate nucleus head, bilateral temporal sulcus gyrus, and parahippocampal gyrus, which also met the laboratory indicators and imaging characteristics of JE. Positive immunoglobulin M antibody test results for serum and cerebrospinal fluid samples from the patient also confirmed recent JEV infection. Treatment of JE mainly involves supportive therapy, including control of intracranial pressure, maintenance of cerebral perfusion pressure, control of seizures, and prevention of complications[7].
JEV and West Nile virus (WNV) both belong to the genus Flavivirus. There are several previous reports of WNV infection following organ transplantation[10-12]. For example, Winston et al[10] described four solid-organ transplant recipients with donor-derived WNV infection from a common donor residing in a region of increased WNV activity. Two of the four transplant recipients died. Testing of the organ donor for WNV infection was not performed as part of the organ donor screening process. JE following liver transplantation has not previously been reported, and the organ donor in the current study was also not tested for JEV infection during the screening process.
One of the most interesting aspects of the current case is the origin of the JEV. In determining this, we considered several possibilities. The first was the transmission of infection via mosquito bites following liver transplantation. However, the patient was admitted to the ICU after surgery, where there was little chance of a mosquito bite. Additionally, there was no epidemic transmission of JEV in Beijing at the time of infection, so it was unlikely to be transmitted via mosquito bites. The second possibility was that the donor was the source of infection. Recent studies have shown that donor-derived infections (DDIs) in the United States occur in < 1% of all transplant procedures. However, despite the low incidence of DDIs, when transmission does occur, it can result in significant morbidity and mortality[13]. In the current case, the donor died of cerebral hemorrhage following traumatic brain injury. Donation occurred 2 d after trauma. No intracranial infection was observed in the donor, and there was no indication of JE. The recipient developed a fever 11 d after transplantation. Given that the incubation period for JE is commonly 4–21 d, donor latent infection cannot be excluded. The third possibility was that the patient contracted the virus prior to surgery and was in the incubation period of infection. After surgery, immunosuppressive agents were administered to the patient. Most immunosuppressive drugs target T lymphocytes, which are the primary mediators of an immunogenic reaction against the graft, leading to rejection. Modern immunosuppressive regimens include two or more drugs that target the immune system at different levels. Higher levels of immunosuppression mean a higher risk of infection, with rates of infection typically highest in the early post-transplantation period[14]. As time passes and the level of immunosuppression is reduced, liver recipients are less prone to infection[15]. Unfortunately, the lack of screening for JEV in the donor and recipient prior to transplantation meant that we were unable to identify the source of infection, which is a weakness of this study.
Conventional screening makes it difficult to detect pathogens such as trypanosomes, human immunodeficiency virus, WNV, hepatitis C virus, Mycobacterium tuberculosis, rabies virus, and multidrug-resistant bacteria. In addition, donor assessment must be completed within a short window of time. The allocation and transportation of organs limit our ability to assess each potential risk. Therefore, transmission events from donors are not completely avoidable[16].
There were some limitations to this case report. There was no cerebrospinal fluid pathogen detection at the onset of the disease, and re-examination should be conducted after recovery with magnetic resonance imaging.
As far as we know, JE following liver transplantation has not previously been reported. Although JE is a serious public health concern with high mortality rate in Asia, this case is unique due to lack of definitely epidemiological contact. We highly suspect it is related to patient’s immunocompromised status. Imaging and lumbar puncture examination should be performed as soon as possible when patients present with fever and central nervous system symptoms post liver transplantation, which is helpful for early diagnosis and improves prognosis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Govindarajan GK S-Editor: Wang YQ L-Editor: Filipodia E-Editor: Liu JH
| 1. | Martin P, DiMartini A, Feng S, Brown R, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 581] [Cited by in F6Publishing: 572] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 2. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 543] [Cited by in F6Publishing: 576] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 3. | Abad CL, Lahr BD, Razonable RR. Epidemiology and risk factors for infection after living donor liver transplantation. Liver Transpl. 2017;23:465-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Kim SI. Bacterial infection after liver transplantation. World J Gastroenterol. 2014;20:6211-6220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 103] [Cited by in F6Publishing: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Zhang H, Wang Y, Li K, Mehmood K, Gui R, Li J. Epidemiology of Japanese Encephalitis in China (2004-2015). Travel Med Infect Dis. 2019;28:109-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Heffelfinger JD, Li X, Batmunkh N, Grabovac V, Diorditsa S, Liyanage JB, Pattamadilok S, Bahl S, Vannice KS, Hyde TB, Chu SY, Fox KK, Hills SL, Marfin AA. Japanese Encephalitis Surveillance and Immunization - Asia and Western Pacific Regions, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:579-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 7. | Tiroumourougane SV, Raghava P, Srinivasan S. Japanese viral encephalitis. Postgrad Med J. 2002;78:205-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Freeman RB, Wiesner RH, Harper A, McDiarmid SV, Lake J, Edwards E, Merion R, Wolfe R, Turcotte J, Teperman L; UNOS/OPTN Liver Disease Severity Score, UNOS/OPTN Liver and Intestine, and UNOS/OPTN Pediatric Transplantation Committees. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 545] [Cited by in F6Publishing: 501] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 9. | Chhabra P, Ranjan P, Bhasin DK. Simultaneous Occurrence of Varicella Zoster Virus-Induced Pancreatitis and Hepatitis in a Renal Transplant Recipient: A Case Report and Review of Literature. Perm J. 2017;21:16-083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Winston DJ, Vikram HR, Rabe IB, Dhillon G, Mulligan D, Hong JC, Busuttil RW, Nowicki MJ, Mone T, Civen R, Tecle SA, Trivedi KK, Hocevar SN; West Nile Virus Transplant-Associated Transmission Investigation Team. Donor-derived West Nile virus infection in solid organ transplant recipients: report of four additional cases and review of clinical, diagnostic, and therapeutic features. Transplantation. 2014;97:881-889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Inojosa WO, Scotton PG, Fuser R, Giobbia M, Paolin A, Maresca MC, Brunello A, Nascimben E, Sorbara C, Rigoli R, Berti R, Gajo GB, Giometto B. West Nile virus transmission through organ transplantation in north-eastern Italy: a case report and implications for pre-procurement screening. Infection. 2012;40:557-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Rabe IB, Schwartz BS, Farnon EC, Josephson SA, Webber AB, Roberts JP, de Mattos AM, Gallay BJ, van Slyck S, Messenger SL, Yen CJ, Bloch EM, Drew CP, Fischer M, Glaser CA; WNV Transplant Investigation Team. Fatal transplant-associated west nile virus encephalitis and public health investigation-california, 2010. Transplantation. 2013;96:463-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Ison MG, Nalesnik MA. An update on donor-derived disease transmission in organ transplantation. Am J Transplant. 2011;11:1123-1130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Chelala L, Kovacs CS, Taege AJ, Hanouneh IA. Common infectious complications of liver transplant. Cleve Clin J Med. 2015;82:773-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601-2614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1386] [Cited by in F6Publishing: 1256] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 16. | Zhang JR, Sun LY, Zhu ZJ, Wei L, Zeng ZG, Qu W, Liu Y, Song W, Zhang L, He EH, Xu RF, Fang L. The effect of donor-derived infections on liver transplantation recipents. Shiyong Qiguan Yizhi Dianzizazhi. 2018;6:17-20. [DOI] [Cited in This Article: ] |