Abstract
Background
Cancer antigen 19-9 (CA19-9) is widely used as a marker of pancreatic cancer tumor burden and response to therapy. Synthesis of CA19-9 and its circulating levels are determined by variants encoding the fucosyltransferases, FUT2 and FUT3. Individuals can be grouped into one of four functional FUT groups (FUT3-null, FUT-low, FUT-intermediate, FUT-high), each with its own CA19-9 reference range based on its predicted capacity to produce CA19-9. The authors hypothesized that a FUT variant-based CA19-9 tumor marker gene test could improve the prognostic performance of CA19-9.
Methods
Preoperative and pre-treatment CA19-9 levels were measured, and FUT variants were determined in 449 patients who underwent surgery for pancreatic ductal adenocarcinoma (PDAC) at Johns Hopkins Hospital between 2010 and 2020, including 270 patients who underwent neoadjuvant therapy. Factors associated with recurrence-free and overall survival were determined in Cox proportional hazards models.
Results
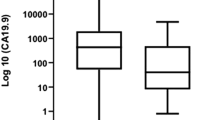
Higher preoperative CA19-9 levels were associated with recurrence and mortality for patients in the higher-FUT groups (FUT-intermediate, FUT-high for mortality, with adjustment for other prognostic factors; hazard ratio [HR], 1.34 and 1.58, respectively; P < 0.001), but not for those in the lower-FUT groups (FUT3-null, FUT-low). As a tumor marker, CA19-9 levels of 100 U/ml or lower after neoadjuvant therapy and normalization of CA19-9 based on FUT group were more sensitive but less specific predictors of evidence for a major pathologic response to therapy (little/no residual tumor) and of early recurrence (within 6 months).
Conclusion
Among patients undergoing pancreatic cancer resection, a CA19-9 tumor marker gene test modestly improved the prognostic performance of CA19-9.

Similar content being viewed by others
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. https://doi.org/10.3322/caac.21763.
Belfiori G, Crippa S, Francesca A, et al. Long-term survivors after upfront resection for pancreatic ductal adenocarcinoma: an actual 5-year analysis of disease-specific and post-recurrence survival. Ann Surg Oncol. 2021;28:8249–60. https://doi.org/10.1245/s10434-10021-10401-10437.
Rangelova E, Wefer A, Persson S, et al. Surgery improves survival after neoadjuvant therapy for borderline and locally advanced pancreatic cancer: a single-institution experience. Ann Surg. 2021;273:579–86. https://doi.org/10.1097/SLA.0000000000003301.
Versteijne E, van Dam JL, Suker M, et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the Dutch randomized PREOPANC trial. J Clin Oncol. 2022;40:1220–30. https://doi.org/10.1200/JCO.1221.02233.
Blinn P, Shridhar R, Maramara T, Huston J, Meredith K. Multi-agent neoadjuvant chemotherapy improves response and survival in patients with resectable pancreatic cancer. J Gastrointest Oncol. 2020;11:1078–89. https://doi.org/10.21037/jgo.22019.21012.21003.
van Dam JL, Janssen QP, Besselink MG, et al. Neoadjuvant therapy or upfront surgery for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomised controlled trials. Eur J Cancer. 2022;160:140–9. https://doi.org/10.1016/j.ejca.2021.1010.1023.
Ushida Y, Inoue Y, Ito H, et al. High CA19-9 level in resectable pancreatic cancer is a potential indication of neoadjuvant treatment. Pancreatology. 2021;21:130–7. https://doi.org/10.1016/j.pan.2020.1011.1026.
Azizian A, Rühlmann F, Krause T, et al. CA19-9 for detecting recurrence of pancreatic cancer. Sci Rep. 2020;10:1332. https://doi.org/10.1038/s41598-41020-57930-x.
Humphris JL, Chang DK, Johns AL, et al. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol. 2012;23:1713–22. https://doi.org/10.1093/annonc/mdr1561.
Soloff EV, Al-Hawary MM, Desser TS, Fishman EK, Minter RM, Zins M. Imaging assessment of pancreatic cancer resectability after neoadjuvant therapy: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218:570–81. https://doi.org/10.2214/AJR.2221.26931.
Al Abbas AI, Zenati M, Reiser CJ, et al. Serum CA19-9 response to neoadjuvant therapy predicts tumor size reduction and survival in pancreatic adenocarcinoma. Ann Surg Oncol. 2020;27:2007–14. https://doi.org/10.1245/s10434-10019-08156-10433.
Tsai S, George B, Wittmann D, et al. Importance of normalization of CA19-9 levels following neoadjuvant therapy in patients with localized pancreatic cancer. Ann Surg. 2020;271:740–7. https://doi.org/10.1097/SLA.0000000000003049.
Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18:2–11. https://doi.org/10.1016/j.pan.2017.1011.1011.
Doppenberg D, van Dam JL, Han Y, et al. Predictive value of baseline serum carbohydrate antigen 19–9 level on treatment effect of neoadjuvant chemoradiotherapy in patients with resectable and borderline resectable pancreatic cancer in two randomized trials. Br J Surg. 2023;110(10):1374–80.
Tempero MA, Pelzer U, O’Reilly EM, et al. Adjuvant nab-paclitaxel + gemcitabine in resected pancreatic ductal adenocarcinoma: results from a randomized, open-label, phase III trial. J Clin Oncol. 2022;15:01134.
Kawai S, Suzuki K, Nishio K, et al. Smoking and serum CA19-9 levels according to Lewis and secretor genotypes. Int J Cancer. 2008;123:2880–4.
Luo G, Guo M, Jin K, et al. Optimize CA19-9 in detecting pancreatic cancer by Lewis and secretor genotyping. Pancreatology. 2016;16:1057–62.
Wannhoff A, Hov JR, Folseraas T, et al. FUT2 and FUT3 genotype determines CA19-9 cut-off values for detection of cholangiocarcinoma in patients with primary sclerosing cholangitis. J Hepatol. 2013;59:1278–84.
Abe T, Koi C, Kohi S, et al. Gene variants that affect levels of circulating tumor markers increase identification of patients with pancreatic cancer. Clin Gastroenterol Hepatol. 2020;18:1161-1169.e1165. https://doi.org/10.1016/j.cgh.2019.1110.1036.
Narimatsu H, Iwasaki H, Nakayama F, et al. Lewis and secretor gene dosages affect CA19-9 and DU-PAN-2 serum levels in normal individuals and colorectal cancer patients. Cancer Res. 1998;58:512–8.
Dbouk M, Abe T, Koi C, et al. Diagnostic performance of a tumor marker gene test that personalizes the normal reference range of CA19-9. Clin Cancer Res. 2023;29(20):4178–85.
Washington KBJ BJ, Branton P, Burgart LJ, Carter DK,, Compton CC F. Protocol for the examination of specimens from patients with carcinoma of the pancreas, 2016. Retrieved 25 March 2023 at https://documents.cap.org/protocols/cp-pancreas-exocrine-2016-v3301.pdf.
Janssen BV, Tutucu F, van Roessel S, et al. Amsterdam international consensus meeting: tumor response scoring in the pathology assessment of resected pancreatic cancer after neoadjuvant therapy. Mod Pathol. 2021;34:4–12. https://doi.org/10.1038/s41379-41020-00683-41379.
Shindo K, Yu J, Suenaga M, et al. Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J Clin Oncol. 2017;35:3382–90.
Cakir B, Pankow JS, Salomaa V, et al. Distribution of Lewis (FUT3) genotype and allele: frequencies in a biethnic United States population. Ann Hematol. 2002;81:558–65. https://doi.org/10.1007/s00277-00002-00508-x.
Ferrer-Admetlla A, Sikora M, Laayouni H, et al. A natural history of FUT2 polymorphism in humans. Mol Biol Evol. 2009;26:1993–2003. https://doi.org/10.1093/molbev/msp1108.
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2022. Vienna, Austria. Retrieved January 23, 2024, from https://www.R-project.org/.
Maeda S, Mederos MA, Chawla A, et al. Pathological treatment response has different prognostic implications for pancreatic cancer patients treated with neoadjuvant chemotherapy or chemoradiotherapy. Surgery. 2022;171:1379–87. https://doi.org/10.1016/j.surg.2021.1310.1015.
Seelen LWF, Floortje van Oosten A, Brada LJH, et al. Early recurrence after resection of locally advanced pancreatic cancer following induction therapy: an international multicenter study. Ann Surg. 2022;11:0000000000005666.
Abdelrahman AM, Goenka AH, Alva-Ruiz R, et al. FDG-PET predicts neoadjuvant therapy response and survival in borderline resectable/locally advanced pancreatic adenocarcinoma. J Natl Compr Canc Netw. 2022;20:1023-32.e1023. https://doi.org/10.6004/jnccn.2022.7041.
Groot VP, Blair AB, Gemenetzis G, et al. Recurrence after neoadjuvant therapy and resection of borderline resectable and locally advanced pancreatic cancer. Eur J Surg Oncol. 2019;45:1674–83. https://doi.org/10.1016/j.ejso.2019.1604.1007.
Takagi T, Nagai M, Nishiwada S, et al. Importance of triple tumor markers as biomarkers in patients with pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg. 2023;7:326–35. https://doi.org/10.1002/ags1003.12629.eCollection12023Mar.
Thalji SZ, Kamgar M, George B, et al. CA19-9 response to first-line neoadjuvant FOLFIRINOX and second-line gemcitabine/nab-paclitaxel for patients with operable pancreatic cancer. Ann Surg Oncol. 2023;30(5):3013–21. https://doi.org/10.1245/s10434-022-13055-1.
Nitschke C, Markmann B, Walter P, et al. Peripheral and portal venous KRAS ctDNA detection as independent prognostic markers of early tumor recurrence in pancreatic ductal adenocarcinoma. Clin Chem. 2023;69:295–307. https://doi.org/10.1093/clinchem/hvac1214.
Kitahata Y, Kawai M, Hirono S, et al. Circulating tumor DNA as a potential prognostic marker in patients with borderline-resectable pancreatic cancer undergoing neoadjuvant chemotherapy followed by pancreatectomy. Ann Surg Oncol. 2022;29:1596–605. https://doi.org/10.1245/s10434-10021-10985-10430.
Ecker BL, Tao AJ, Janssen QP, et al. Genomic biomarkers associated with response to induction chemotherapy in patients with localized pancreatic ductal adenocarcinoma. Clin Cancer Res. 2023;29(7):1368–74.
Seppälä TT, Zimmerman JW, Suri R, et al. Precision medicine in pancreatic cancer: patient-derived organoid pharmacotyping is a predictive biomarker of clinical treatment response. Clin Cancer Res. 2022;28:3296–307. https://doi.org/10.1158/1078-0432.CCR-3221-4165.
Motoi F, Kosuge T, Ueno H, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol. 2019;49:190–4. https://doi.org/10.1093/jjco/hyy1190.
Liu H, D’Alesio M, AlMasri S, et al. No survival benefit with suboptimal CA19-9 response: defining effective neoadjuvant chemotherapy in resectable or borderline resectable pancreatic cancer. HPB. 2023;25(5):521–32.
Acknowledgments
This work was supported by NIH grants (U01210170, R01CA176828 CA62924), Susan Wojcicki and Dennis Troper, and a Stand Up to Cancer–Lustgarten Foundation Pancreatic Cancer Interception Translational Cancer Research grant (grant no. SU2C-AACR-DT25-17). Stand Up to Cancer is a program of the Entertainment Industry Foundation. SU2C research grants are administered by the American Association for Cancer Research, the scientific partner of SU2C. Michael Goggins is the Sol Goldman Professor of Pancreatic Cancer Research. A Fujimoto Medical System scholarship was awarded to Yohei Ando and the International Research Fund for Subsidy of Kyushu University School of Medicine Alumni.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ando, Y., Dbouk, M., Blackford, A.L. et al. Using a CA19-9 Tumor Marker Gene Test to Assess Outcome After Pancreatic Cancer Surgery. Ann Surg Oncol 31, 2902–2912 (2024). https://doi.org/10.1245/s10434-024-14942-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-14942-5