Abstract
Background
Surgical resection is the primary treatment for bone and soft tissue tumors. Negative margin status is a key factor in prognosis. Given the three-dimensional (3D) anatomic complexity of musculoskeletal tumor specimens, communication of margin results between surgeons and pathologists is challenging. We sought to perform ex vivo 3D scanning of musculoskeletal oncology specimens to enhance communication between surgeons and pathologists.
Methods
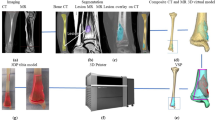
Immediately after surgical resection, 3D scanning of the fresh specimen is performed prior to frozen section analysis. During pathologic grossing, whether frozen or permanent, margin sampling sites are annotated on the virtual 3D model using computer-aided design (CAD) software.
Results
3D scanning was performed in seven cases (six soft tissue, one bone), with specimen mapping on six cases. Intraoperative 3D scanning and mapping was performed in one case in which the location of margin sampling was shown virtually in real-time to the operating surgeon to help achieve a negative margin. In six cases, the 3D model was used to communicate final permanent section analysis. Soft tissue, cartilage, and bone (including lytic lesions within bone) showed acceptable resolution.
Conclusions
Virtual 3D scanning and specimen mapping is feasible and may allow for enhanced documentation and communication. This protocol provides useful information for anatomically complex musculoskeletal tumor specimens. Future studies will evaluate the effect of the protocol on positive margin rates, likelihood that a re-resection contains additional malignancy, and exploration of targeted adjuvant radiation protocols using a patient-specific 3D specimen map.






Similar content being viewed by others
References
He F, Zhang W, Shen Y, et al. Effects of resection margins on local recurrence of osteosarcoma in extremity and pelvis: systematic review and meta-analysis. Int J Surg. 2016;36(Pt A):283–92.
Sambri A, Caldari E, Fiore M, et al. Margin assessment in soft tissue sarcomas: review of the literature. Cancers (Basel). 2021;13(7):1687.
O’Donnell PW, Griffin AM, Eward WC, et al. The effect of the setting of a positive surgical margin in soft tissue sarcoma. Cancer. 2014;120(18):2866–75.
Liu CY, Yen CC, Chen WM, et al. Soft tissue sarcoma of extremities: the prognostic significance of adequate surgical margins in primary operation and reoperation after recurrence. Ann Surg Oncol. 2010;17(8):2102–11.
Traub F, Griffin AM, Wunder JS, Ferguson PC. Influence of unplanned excisions on the outcomes of patients with stage III extremity soft-tissue sarcoma. Cancer. 2018;124(19):3868–75.
Gadgeel SM, Harlan LC, Zeruto CA, Osswald M, Schwartz AG. Patterns of care in a population-based sample of soft tissue sarcoma patients in the United States. Cancer. 2009;115(12):2744–54.
Bertrand TE, Cruz A, Binitie O, Cheong D, Letson GD. Do surgical margins affect local recurrence and survival in extremity, nonmetastatic, high-grade osteosarcoma? Clin Orthopaed Relat Research. 2016;474(3):677–83.
Ahmad R, Jacobson A, Hornicek F, et al. The width of the surgical margin does not influence outcomes in extremity and truncal soft tissue sarcoma treated with radiotherapy. Oncologist. 2016;21(10):1269–76.
Sharif KF, Prasad K, Miller A, et al. Enhanced intraoperative communication of tumor margins using 3D scanning and mapping: the computer-aided design margin. Laryngoscope. 2023;133(8):1914–8.
Miller A, Prasad K, Sharif KF, et al. Virtual 3D specimen mapping in head & neck oncologic surgery. Laryngoscope. Epub 19 Jul 2023. https://doi.org/10.1002/lary.30881.
Sharif KF, Lewis JS Jr, Ely KA, et al. The computer-aided design margin: ex vivo 3D specimen mapping to improve communication between surgeons and pathologists. Head Neck. 2023;45(1):22–31.
Brouwer de Koning SG, Schaeffers A, Schats W, van den Brekel MWM, Ruers TJM, Karakullukcu MB. Assessment of the deep resection margin during oral cancer surgery: a systematic review. Eur J Surg Oncol. 2021;47(9):2220–32.
Boyd J, Jonard B, Weiner S. Desmoplastic fibroma in the distal humerus of a 14-year-old boy: a case report. JBJS Case Connect. 2019;9(4):e0155.
Tanwar YS, Kharbanda Y, Rastogi R, Singh R. Desmoplastic fibroma of bone: a case series and review of literature. Indian J Surg Oncol. 2018;9(4):585–91.
Evans S, Ramasamy A, Jeys L, Grimer R. Desmoplastic fibroma of bone: a rare bone tumour. J Bone Oncol. 2014;3(3–4):77–9.
Papke DJ, Hung YP, Schaefer IM, et al. Clinicopathologic characterization of malignant chondroblastoma: a neoplasm with locally aggressive behavior and metastatic potential that closely mimics chondroblastoma-like osteosarcoma. Mod Pathol. 2020;33(11):2295–306.
Visgauss JD, Lazarides A, Dickson B, et al. Treatment of chondroblastoma with denosumab: a case report with a correlative analysis of effect on the RANK signaling pathway. JBJS Case Connect. 2021;11(2):e20.
Focaccia M, Gambarotti M, Hakim R, et al. Chondroblastoma’s lung metastases treated with denosumab in pediatric patient. Cancer Res Treat. 2021;53(1):279–82.
Suster DI, Kurzawa P, Neyaz A, et al. Chondroblastoma expresses RANKL by RNA in situ hybridization and may respond to denosumab therapy. Am J Surg Pathol. 2020;44(12):1581–90.
Gao RW, Teraphongphom NT, van den Berg NS, et al. Determination of tumor margins with surgical specimen mapping using near-infrared fluorescence. Cancer Res. 2018;78(17):5144–54.
Tummers WS, Warram JM, van den Berg NS, et al. Recommendations for reporting on emerging optical imaging agents to promote clinical approval. Theranostics. 2018;8(19):5336–47.
VanKoevering KK, Hollister SJ, Green GE. Advances in 3-dimensional printing in otolaryngology: a review. JAMA Otolaryngol Head Neck Surg. 2017;143(2):178–83.
de Boer E, Moore LS, Warram JM, et al. On the horizon: Optical imaging for cutaneous squamous cell carcinoma. Head Neck. 2016;38(Suppl 1):E2204-2213.
de Boer E, Harlaar NJ, Taruttis A, et al. Optical innovations in surgery. Br J Surg. 2015;102(2):e56-72.
Farahani N, Braun A, Jutt D, et al. Three-dimensional imaging and scanning: current and future applications for pathology. J Pathol Inform. 2017;8:36.
Harmsen S, Teraphongphom N, Tweedle MF, Basilion JP, Rosenthal EL. Optical surgical navigation for precision in tumor resections. Mol Imaging Biol. 2017;19(3):357–62.
Keshtgar MR, Chicken DW, Austwick MR, et al. Optical scanning for rapid intraoperative diagnosis of sentinel node metastases in breast cancer. Br J Surg. 2010;97(8):1232–9.
van Keulen S, Nishio N, Fakurnejad S, et al. the clinical application of fluorescence-guided surgery in head and neck cancer. J Nucl Med. 2019;60(6):758–63.
Nguyen JQ, Gowani ZS, O’Connor M, et al. Intraoperative Raman spectroscopy of soft tissue sarcomas. Lasers Surg Med. 2016;48(8):774–81.
Kerawala CJ, Ong TK. Relocating the site of frozen sections–is there room for improvement? Head Neck. 2001;23(3):230–2.
Coutu B, Ryan E, Christensen D, et al. Positive margins matter regardless of subsequent resection findings. Oral Oncol. 2022;128:105850.
Prasad K, Sharma R, Habib D, et al. How often is cancer present in oral cavity re-resections after initial positive margins? Laryngoscope. Epub 16 Aug 2023. https://doi.org/10.1002/lary.30959.
Ettl T, El-Gindi A, Hautmann M, et al. Positive frozen section margins predict local recurrence in R0-resected squamous cell carcinoma of the head and neck. Oral Oncol. 2016;55:17–23.
Buchakjian MR, Ginader T, Tasche KK, Pagedar NA, Smith BJ, Sperry SM. Independent predictors of prognosis based on oral cavity squamous cell carcinoma surgical margins. Otolaryngol Head Neck Surg. 2018;159(4):675–82.
Priya SR, D’Cruz AK, Pai PS. Cut margins and disease control in oral cancers. J Cancer Res Therap. 2012;8(1):74–9.
Haas RL, Delaney TF, O’Sullivan B, et al. Radiotherapy for management of extremity soft tissue sarcomas: why, when, and where? Int J Radiat Oncol Biol Phys. 2012;84(3):572–80.
Mills GL. CORR Insights®: ninety percent or greater tumor necrosis is associated with survival and social determinants of health in patients with osteosarcoma in the national cancer database. Clin Orthop Relat Res. 2023;481(3):523–5.
Richardson SM, Wurtz LD, Collier CD. Ninety percent or greater tumor necrosis is associated with survival and social determinants of health in patients with osteosarcoma in the national cancer database. Clin Orthop Relat Res. 2023;481(3):512–22.
Tsuda Y, Tsoi K, Parry MC, et al. Impact of chemotherapy-induced necrosis on event-free and overall survival after preoperative MAP chemotherapy in patients with primary high-grade localized osteosarcoma. Bone Joint J. 2020;102-B(6):795–803.
Simon MA, Hecht JD. Invasion of joints by primary bone sarcomas in adults. Cancer. 1982;50(8):1649–55.
Acknowledgment
JMC is supported by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health under award number T32GM007347. The content in this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
This work was supported by a Vanderbilt Clinical Oncology Research Career Development Program (K12 NCI 2K12CA090625-22A1) and an ACS Institutional Research Grant (#IRG-19-139-60).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Kayvon Sharif: USPTO application number: 63/351,292. Patent application title: Three-Dimensional Models of Surgical Margins. Juan M. Colazo, Kavita Prasad, Alexis Miller, Marina Aweeda, Carly Fassler, Reena Singh, Herbert S. Schwartz, Joshua M. Lawrenz, Ginger E. Holt, and Michael C. Topf have no conflicts of interest to declare in relation to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 1292 KB)
Supplementary file3 (MP4 9912 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Colazo, J.M., Prasad, K., Miller, A. et al. 3D Specimen Scanning and Mapping in Musculoskeletal Oncology: A Feasibility Study. Ann Surg Oncol 31, 2051–2060 (2024). https://doi.org/10.1245/s10434-023-14757-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14757-w