Abstract
Background
Intraoperative specimen mammography is a valuable tool in breast cancer surgery, providing immediate assessment of margins for a resected tumor. However, the accuracy of specimen mammography in detecting microscopic margin positivity is low. We sought to develop an artificial intelligence model to predict the pathologic margin status of resected breast tumors using specimen mammography.
Methods
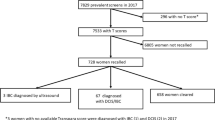
A dataset of specimen mammography images matched with pathologic margin status was collected from our institution from 2017 to 2020. The dataset was randomly split into training, validation, and test sets. Specimen mammography models pretrained on radiologic images were developed and compared with models pretrained on nonmedical images. Model performance was assessed using sensitivity, specificity, and area under the receiver operating characteristic curve (AUROC).
Results
The dataset included 821 images, and 53% had positive margins. For three out of four model architectures tested, models pretrained on radiologic images outperformed nonmedical models. The highest performing model, InceptionV3, showed sensitivity of 84%, specificity of 42%, and AUROC of 0.71. Model performance was better among patients with invasive cancers, less dense breasts, and non-white race.
Conclusions
This study developed and internally validated artificial intelligence models that predict pathologic margins status for partial mastectomy from specimen mammograms. The models’ accuracy compares favorably with published literature on surgeon and radiologist interpretation of specimen mammography. With further development, these models could more precisely guide the extent of resection, potentially improving cosmesis and reducing reoperations.




Similar content being viewed by others
References
Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–41. https://doi.org/10.1056/nejmoa022152.
Marinovich ML, Azizi L, Macaskill P, et al. The association of surgical margins and local recurrence in women with ductal carcinoma in situ treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol. 2016;23(12):3811–21. https://doi.org/10.1245/s10434-016-5446-2.
Morrow M, Abrahamse P, Hofer TP, et al. Trends in reoperation after initial lumpectomy for breast cancer addressing overtreatment in surgical management author audio interview supplemental content. JAMA Oncol. 2017;3(10):1352–7. https://doi.org/10.1001/jamaoncol.2017.0774.
Moran MS, Schnitt SJ, Giuliano AE, et al. SSO-ASTRO consensus guideline on margins for breast-conserving surgery with whole breast irradiation in stage I and II invasive breast cancer. Int J Radiat Oncol Biol Phys. 2014;88(3):553. https://doi.org/10.1016/J.IJROBP.2013.11.012.
Houssami N, Macaskill P, Luke Marinovich M, Morrow M. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: A meta-analysis. Ann Surg Oncol. 2014;21(3):717–30. https://doi.org/10.1245/s10434-014-3480-5.
Rosenberger LH, Mamtani A, Fuzesi S, et al. Early adoption of the SSO-ASTRO consensus guidelines on margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: initial experience from Memorial Sloan Kettering Cancer Center. Ann Surg Oncol. 2016;23(10):3239–46. https://doi.org/10.1245/S10434-016-5397-7.
Versteegden DPA, Keizer LGG, Schlooz-Vries MS, Duijm LEM, Wauters CAP, Strobbe LJA. Performance characteristics of specimen radiography for margin assessment for ductal carcinoma in situ: a systematic review. Breast Cancer Res Treat. 2017;166(3):669–79. https://doi.org/10.1007/s10549-017-4475-2.
Layfield DM, May DJ, Cutress RI, et al. The effect of introducing an in-theatre intra-operative specimen radiography (IOSR) system on the management of palpable breast cancer within a single unit. Breast. 2012;21(4):459–63. https://doi.org/10.1016/j.breast.2011.10.010.
Bathla L, Harris A, Davey M, Sharma P, Silva E. High resolution intra-operative two-dimensional specimen mammography and its impact on second operation for re-excision of positive margins at final pathology after breast conservation surgery. Am J Surg. 2011;202:387–94. https://doi.org/10.1016/j.amjsurg.2010.09.031.
Hisada T, Sawaki M, Ishiguro J, et al. Impact of intraoperative specimen mammography on margins in breast-conserving surgery. Mol Clin Oncol. 2016;5(3):269–72. https://doi.org/10.3892/mco.2016.948.
Funk A, Heil J, Harcos A, et al. Efficacy of intraoperative specimen radiography as margin assessment tool in breast conserving surgery. Breast Cancer Res Treat. 2020;179(2):425–33. https://doi.org/10.1007/s10549-019-05476-6.
Yen TWF, Pezzin LE, Li J, Sparapani R, Laud PW, Nattinger AB. Effect of hospital volume on processes of breast cancer care: a National Cancer Data Base study. Cancer. 2017;123(6):957–66. https://doi.org/10.1002/CNCR.30413.
Chagpar AB, Killelea BK, Tsangaris TN, et al. A randomized, controlled trial of cavity shave margins in breast cancer. N Engl J Med. 2015;373(6):503–10. https://doi.org/10.1056/NEJMoa1504473.
Dupont E, Tsangaris T, Garcia-Cantu C, et al. Resection of cavity shave margins in stage 0–III breast cancer patients undergoing breast conserving surgery. Ann Surg. 2019. https://doi.org/10.1097/sla.0000000000003449.
Cartagena LC, McGuire K, Zot P, Pillappa R, Idowu M, Robila V. Breast-conserving surgeries with and without cavity shave margins have different re-excision rates and associated overall cost: institutional and patient-driven decisions for its utilization. Clin Breast Cancer. 2021;21(5):e594–601. https://doi.org/10.1016/J.CLBC.2021.03.003.
Lecun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436–44. https://doi.org/10.1038/nature14539.
Aggarwal R, Sounderajah V, Martin G, et al. Diagnostic accuracy of deep learning in medical imaging: a systematic review and meta-analysis. NPJ Digit Med. 2021;4(1):1–23. https://doi.org/10.1038/s41746-021-00438-z.
Yala A, Lehman C, Schuster T, Portnoi T, Barzilay R. A deep learning mammography-based model for improved breast cancer risk prediction. Radiology. 2019;292(1):60–6. https://doi.org/10.1148/radiol.2019182716.
Lehman CD, Yala A, Schuster T, et al. Mammographic breast density assessment using deep learning: Clinical implementation. Radiology. 2019;290(1):52–8. https://doi.org/10.1148/radiol.2018180694.
Hashimoto DA, Rosman G, Witkowski ER, et al. Computer vision analysis of intraoperative video: automated recognition of operative steps in laparoscopic sleeve gastrectomy. Ann Surg. 2019;270(3):414–21. https://doi.org/10.1097/SLA.0000000000003460.
Radiology AC of, D’Orsi CJ. ACR BI-RADS Atlas: Breast Imaging Reporting and Data System: 2013. Published online 2018. Accessed May 3, 2023. https://books.google.com/books/about/2013_ACR_BI_RADS_Atlas.html?id=nhWSjwEACAAJ
Gradishar WJ, Anderson BO, Abraham J, et al. Breast cancer, version 32020. JNCCN J Natl Compr Cancer Netw. 2020;18(4):452–78. https://doi.org/10.6004/jnccn.2020.0016.
Zheng A, Casari A. Feature Engineering for Machine Learning PRINCIPLES AND TECHNIQUES FOR DATA SCIENTISTS.
Mei X, Liu Z, Robson PM, Marinelli B, Huang M, Doshi A, Jacobi A, Cao C, Link KE, Yang T, Wang Y. RadImageNet: an open radiologic deep learning research dataset for effective transfer learning. Radiol Artif Intell. 2022;4(5):e210315. https://doi.org/10.1148/ryai.210315.
Russakovsky O, Deng J, Su H, et al. ImageNet Large scale visual recognition challenge. Int J Comput Vis. 2015;115(3):211–52. https://doi.org/10.1007/s11263-015-0816-y.
Yala A, Mikhael PG, Strand F, et al. Multi-institutional validation of a mammography-based breast cancer risk model. J Clin Oncol. 2022;40(16):1732–40. https://doi.org/10.1200/JCO.21.01337.
Siu AL. Screening for breast cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2016;164(4):279–96. https://doi.org/10.7326/M15-2886.
Selvaraju RR, Cogswell M, Das A, Vedantam R, Parikh D, Batra D. Grad-CAM: visual explanations from deep networks via gradient-based localization. Int J Comput Vis. 2016;128(2):336–59. https://doi.org/10.1007/s11263-019-01228-7.
Pedregosa F, Varoquaux G, Gramfort A, et al. Scikit-learn: machine learning in Python. J Mach Learn Res. 2011;12:2825–30.
Chollet F, others. Keras. Published online 2015. https://github.com/fchollet/keras
Pollard TJ, Johnson AEW, Raffa JD, Mark RG. tableone: An open source Python package for producing summary statistics for research papers. JAMIA Open. 2018;1(1):26. https://doi.org/10.1093/JAMIAOPEN/OOY012.
Landercasper J, Attai D, Atisha D, et al. Toolbox to reduce lumpectomy reoperations and improve cosmetic outcome in breast cancer patients: The American Society of Breast Surgeons Consensus Conference. Ann Surg Oncol. 2015;22(10):3174. https://doi.org/10.1245/S10434-015-4759-X.
St John ER, Al-Khudairi R, Ashrafian H, et al. Diagnostic accuracy of intraoperative techniques for margin assessment in breast cancer surgery a meta-analysis. Ann Surg. 2017;265(2):300–10. https://doi.org/10.1097/SLA.0000000000001897.
Kulkarni SA, Kulkarni K, Schacht D, et al. High-resolution full-3D specimen imaging for lumpectomy margin assessment in breast cancer. Ann Surg Oncol. 2021;28(10):5513–24. https://doi.org/10.1245/s10434-021-10499-9.
Mazouni C, Rouzier R, Balleyguier C, et al. Specimen radiography as predictor of resection margin status in non-palpable breast lesions. Clin Radiol. 2006;61(9):789–96. https://doi.org/10.1016/j.crad.2006.04.017.
Mario J, Venkataraman S, Fein-Zachary V, Knox M, Brook A, Slanetz P. Lumpectomy specimen radiography: does orientation or 3-dimensional tomosynthesis improve margin assessment? Can Assoc Radiol J. 2019;70(3):282–91. https://doi.org/10.1016/j.carj.2019.03.005.
Manhoobi IP, Bodilsen A, Nijkamp J, et al. Diagnostic accuracy of radiography, digital breast tomosynthesis, micro-CT and ultrasound for margin assessment during breast surgery: A systematic review and meta-analysis. Acad Radiol. 2022;29(10):1560–72. https://doi.org/10.1016/J.ACRA.2021.12.006.
Lange M, Reimer T, Hartmann S. Glass, Stachs A. The role of specimen radiography in breast-conserving therapy of ductal carcinoma in situ. Breast. 2016;26:73–9. https://doi.org/10.1016/j.breast.2015.12.014.
Kim HE, Kim HH, Han BK, et al. Changes in cancer detection and false-positive recall in mammography using artificial intelligence: a retrospective, multireader study. Lancet Digit Heal. 2020;2(3):e138–48. https://doi.org/10.1016/S2589-7500(20)30003-0.
Rodriguez-Ruiz A, Lång K, Gubern-Merida A, et al. Stand-alone artificial intelligence for breast cancer detection in mammography: comparison with 101 radiologists. J Natl Cancer Inst. 2019;111(9):916–22. https://doi.org/10.1093/JNCI/DJY222.
Rodríguez-Ruiz A, Krupinski E, Mordang JJ, et al. Detection of breast cancer with mammography: Effect of an artificial intelligence support system. Radiology. 2019;290(3):305–14. https://doi.org/10.1148/radiol.2018181371.
Madani A, Namazi B, Altieri MS, et al. Artificial intelligence for intraoperative guidance: using semantic segmentation to identify surgical anatomy during laparoscopic cholecystectomy. Ann Surg. 2022;276(2):363–9. https://doi.org/10.1097/SLA.0000000000004594.
Kitaguchi D, Takeshita N, Matsuzaki H, Takano H, Owada Y, Enomoto T, Oda T, Miura H, Yamanashi T, Watanabe M, Sato D. Real-time automatic surgical phase recognition in laparoscopic sigmoidectomy using the convolutional neural network-based deep learning approach. Surg Endosc. 2020;34:4924–31. https://doi.org/10.1007/s00464-019-07281-0.
Cheng K, You J, Wu S, et al. Artificial intelligence-based automated laparoscopic cholecystectomy surgical phase recognition and analysis. Surg Endosc. 2022;36(5):3160–8. https://doi.org/10.1007/s00464-021-08619-3.
Istasy P, Lee WS, Iansavichene A, et al. The impact of artificial intelligence on health equity in oncology: scoping review. J Med Internet Res. 2022;24(11):e39748. https://doi.org/10.2196/39748.
Istasy P, Lee WS, Iansavitchene A, et al. The impact of artificial intelligence on health equity in oncology: a scoping review. Blood. 2021;138(Supplement 1):4934. https://doi.org/10.1182/BLOOD-2021-149264.
Ghorbani A, Wexler Google Brain J, Zou J, Kim Google Brain B. Towards Automatic Concept-Based Explanations. https://github.com/amiratag/ACE
Sercan¨ S, Arık S, Pfister T. Protoattend: Attention-Based Prototypical Learning.
Partain N, Calvo C, Mokdad A, et al. Differences in re-excision rates for breast-conserving surgery using intraoperative 2D versus 3D tomosynthesis specimen radiograph. Ann Surg Oncol. 2020;27(12):4767–76. https://doi.org/10.1245/s10434-020-08877-w.
Un Park K, Kuerer HM, Rauch GM, et al. Digital breast tomosynthesis for intraoperative margin assessment during breast-conserving surgery. Published Online. 2019. https://doi.org/10.1245/s10434-019-07226-w.
Polat YD, Taşkın F, Çildağ MB, Tanyeri A, Soyder A, Ergin F. The role of tomosynthesis in intraoperative specimen evaluation. Breast J. 2018;24(6):992–6. https://doi.org/10.1111/TBJ.13070.
Sandor MF, Schwalbach B, Hofmann V, et al. Imaging of lumpectomy surface with large field-of-view confocal laser scanning microscope for intraoperative margin assessment - POLARHIS study. Breast. 2022;66:118–25. https://doi.org/10.1016/J.BREAST.2022.10.003.
Aref MH, El-Gohary M, Elrewainy A, et al. Emerging technology for intraoperative margin assessment and post-operative tissue diagnosis for breast-conserving surgery. Photodiagnosis Photodyn Ther. 2023. https://doi.org/10.1016/J.PDPDT.2023.103507.
Mondal S, Sthanikam Y, Kumar A, et al. Mass spectrometry imaging of lumpectomy specimens deciphers diacylglycerols as potent biomarkers for the diagnosis of breast cancer. Anal Chem. 2023. https://doi.org/10.1021/ACS.ANALCHEM.3C01019.
Wang J, Zhang L, Pan Z. Evaluating the impact of radiofrequency spectroscopy on reducing reoperations after breast conserving surgery A meta-analysis. Thorac Cancer. 2023. https://doi.org/10.1111/1759-7714.14890.
Zúñiga WC, Jones V, Anderson SM, Echevarria A, Miller NL, Stashko C, Schmolze D, Cha PD, Kothari R, Fong Y, Storrie-Lombardi MC. Raman spectroscopy for rapid evaluation of surgical margins during breast cancer lumpectomy. Scientific reports. 2019;9(1):14639. https://doi.org/10.1038/S41598-019-51112-0.
Schnabel F, Boolbol SK, Gittleman M, et al. A randomized prospective study of lumpectomy margin assessment with use of marginprobe in patients with nonpalpable breast malignancies. Ann Surg Oncol. 2014;21(5):1589–95. https://doi.org/10.1245/s10434-014-3602-0.
Acknowledgement
This work was supported by funding from the National Institutes of Health (Program in Translational Medicine T32-CA244125 to UNC/K.A.C.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure
K.A.C., K.K.G., and S.M.G. hold a preliminary patent describing the methods used in this study. This work was supported by funding from the National Institutes of Health (Program in Translational Medicine T32-CA244125 to UNC/K.A.C.).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A previous version of this project was presented at the American College of Surgeons Clinical Congress 2021.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, K.A., Kirchoff, K.E., Butler, L.R. et al. Analysis of Specimen Mammography with Artificial Intelligence to Predict Margin Status. Ann Surg Oncol 30, 7107–7115 (2023). https://doi.org/10.1245/s10434-023-14083-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14083-1