Abstract
Objectives
We used a novel combined analysis to evaluate various factors associated with failure to surgical resection in non-metastatic gastric cancer.
Methods
We identified factors associated with the receipt of surgery in publicly available clinical trial data for gastric cancer and in the National Cancer Database (NCDB) for patients with stages I–III gastric adenocarcinoma. Next, we evaluated variable importance in predicting the receipt of surgery in the NCDB.
Results
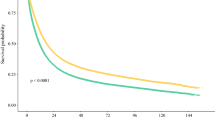
In published clinical trial data, 10% of patients in surgery-first arms did not undergo surgery, mostly due to disease progression and 15% of patients in neoadjuvant therapy arms failed to reach surgery. Effects related to neoadjuvant administration explained the increased attrition (5%). In the NCDB, 61.7% of patients underwent definitive surgery. In a subset of NCDB patients resembling those enrolled in clinical trials (younger, healthier, and privately insured patients treated at high-volume and academic centers) the rate of surgery was 79.2%. Decreased likelihood of surgery was associated with advanced age (OR 0.97, p < 0.01), Charlson–Deyo score of 2+ (OR 0.90, p < 0.01), T4 tumors (OR 0.39, p < 0.01), N+ disease (OR 0.84, p < 0.01), low socioeconomic status (OR 0.86, p = 0.01), uninsured or on Medicaid (OR 0.58 and 0.69, respectively, p < 0.01), low facility volume (OR 0.64, p < 0.01), and non-academic cancer programs (OR 0.79, p < 0.01).
Conclusion
Review of clinical trials shows attrition due to unavoidable tumor and treatment factors (~ 15%). The NCDB indicates non-medical patient and provider characteristics (i.e., age, insurance status, facility volume) associated with attrition. This combined analysis highlights specific opportunities for improving potentially curative surgery rates.


Similar content being viewed by others
Availability of data and material
Supplementary table and publicly available clinical trial data and the de-identified National Cancer Database are available upon request.
References
Hood CM, Gennuso KP, Swain GR, Catlin BB. County health rankings. Am J Prev Med. 2016;50(2):129–35. https://doi.org/10.1016/j.amepre.2015.08.024.
Devarapalli P, Labani S, Nagarjuna N, Panchal P, Asthana S. Barriers affecting uptake of cervical cancer screening in low and middle income countries: a systematic review. Indian J Cancer. 2018;55(4):318–26. https://doi.org/10.4103/ijc.IJC_253_18.
Swords DS, Mulvihill SJ, Brooke BS, Skarda DE, Firpo MA, Scaife CL. Disparities in utilization of treatment for clinical stage I-II pancreatic adenocarcinoma by area socioeconomic status and race/ethnicity. Surgery. 2019;165(4):751–9. https://doi.org/10.1016/j.surg.2018.10.035.
Swords DS, Mulvihill SJ, Brooke BS, Firpo MA, Scaife CL. Size and importance of socioeconomic status-based disparities in use of surgery in nonadvanced stage gastrointestinal cancers. Ann Surg Oncol. 2020;27(2):333–41. https://doi.org/10.1245/s10434-019-07922-7.
Osterman E, Glimelius B. Recurrence risk after up-to-date colon cancer staging, surgery, and pathology: analysis of the entire Swedish population. Dis Colon Rectum. 2018;61(9):1016–25. https://doi.org/10.1097/DCR.0000000000001158.
Stahl KA, Olecki EJ, Dixon ME, et al. Gastric cancer treatments and survival trends in the United States. Curr Oncol. 2020;28(1):138–51. https://doi.org/10.3390/curroncol28010017.
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. https://doi.org/10.1056/NEJMoa055531.
Elshami M, Ahmed FA, Kakish H, et al. Average treatment effect of facility hepatopancreatobiliary cancer volume on survival of non-resected pancreatic adenocarcinoma. HPB (Oxford). 2022;24(11):1878–87. https://doi.org/10.1016/j.hpb.2022.07.007.
Derksen S, Keselman HJ. Backward, forward and stepwise automated subset selection algorithms: frequency of obtaining authentic and noise variables. Br J Math Stat Psychol. 1992;45(2):265–82. https://doi.org/10.1111/j.2044-8317.1992.tb00992.x.
Schechtman E, Schechtman G. The relationship between Gini methodology and the ROC curve. SSRN Electron J. 2016. https://doi.org/10.2139/ssrn.2739245.
Dong W, Bensken WP, Kim U, Rose J, Berger NA, Koroukian SM. Phenotype discovery and geographic disparities of late-stage breast cancer diagnosis across U.S. counties: a machine learning approach. Cancer Epidemiol Biomark Prev. 2022;31(1):66–76. https://doi.org/10.1158/1055-9965.EPI-21-0838.
Boevers E, McDowell BD, Mott SL, Button AM, Lynch CF. Insurance status is related to receipt of therapy and survival in patients with early-stage pancreatic exocrine carcinoma. J Cancer Epidemiol. 2017;2017:1–5. https://doi.org/10.1155/2017/4354592.
Ahmed FA, Elshami M, Hue JJ, et al. Disparities in treatment and survival for patients with isolated colorectal liver metastases. Surgery. 2022. https://doi.org/10.1016/j.surg.2022.09.026.
Liu JH, Zingmond DS, McGory ML, et al. Disparities in the utilization of high-volume hospitals for complex surgery. JAMA. 2006;296(16):1973–80. https://doi.org/10.1001/jama.296.16.1973.
Resio BJ, Chiu AS, Hoag JR, et al. Motivators, barriers, and facilitators to traveling to the safest hospitals in the united states for complex cancer surgery. JAMA Netw Open. 2018;1(7):e184595. https://doi.org/10.1001/jamanetworkopen.2018.4595.
Kaelberer Z, Ruan M, Lam MB, Brindle M, Molina G. Medicaid expansion and surgery for HPB/GI cancers: NCDB difference-in-difference analysis. Am J Surg. 2022. https://doi.org/10.1016/j.amjsurg.2022.09.004.
Hoehn RS, Rieser CJ, Phelos H, et al. Association between medicaid expansion and diagnosis and management of colon cancer. J Am Coll Surg. 2021;232(2):146-156.e1. https://doi.org/10.1016/j.jamcollsurg.2020.10.021.
van Putten M, Nelen SD, Lemmens VEPP, et al. Overall survival before and after centralization of gastric cancer surgery in the Netherlands. Br J Surg. 2018;105(13):1807–15. https://doi.org/10.1002/bjs.10931.
Nelen SD, Heuthorst L, Verhoeven RHA, et al. Impact of centralizing gastric cancer surgery on treatment, morbidity, and mortality. J Gastrointest Surg. 2017;21(12):2000–8. https://doi.org/10.1007/s11605-017-3531-x.
Teh SH, Uong S, Lin TY, et al. Clinical outcomes following regionalization of gastric cancer care in a US integrated health care system. J Clin Oncol. 2021;39(30):3364–76. https://doi.org/10.1200/JCO.21.00480.
Luijten JCHBM, Nieuwenhuijzen GAP, Sosef MN, et al. Impact of nationwide centralization of oesophageal, gastric, and pancreatic surgery on travel distance and experienced burden in the Netherlands. Eur J Surg Oncol. 2022;48(2):348–55. https://doi.org/10.1016/j.ejso.2021.07.023.
Rahouma M, Harrison S, Kamel M, et al. Consequences of refusing surgery for esophageal cancer: a national cancer database analysis. Ann Thorac Surg. 2018;106(5):1476–83. https://doi.org/10.1016/j.athoracsur.2018.06.030.
Pathak R, Canavan ME, Walters S, Salazar MC, Boffa DJ. Chemoradiation as a nonsurgical treatment option for early-stage esophageal cancers: a retrospective cohort study. J Thorac Dis. 2021;13(1):140–8. https://doi.org/10.21037/jtd-20-1187.
Swords DS, Mulvihill SJ, Skarda DE, et al. Hospital-level variation in utilization of surgery for clinical stage I-II pancreatic adenocarcinoma. Ann Surg. 2019;269(1):133–42. https://doi.org/10.1097/SLA.0000000000002404.
Gaitanidis A, Alevizakos M, Tsalikidis C, Tsaroucha A, Simopoulos C, Pitiakoudis M. Refusal of cancer-directed surgery by breast cancer patients: risk factors and survival outcomes. Clin Breast Cancer. 2018;18(4):e469–76. https://doi.org/10.1016/j.clbc.2017.07.010.
Rapp J, Tuminello S, Alpert N, Flores RM, Taioli E. Disparities in surgery for early-stage cancer: the impact of refusal. Cancer Causes Control. 2019;30(12):1389–97. https://doi.org/10.1007/s10552-019-01240-9.
Coffman AR, Tao R, Cohan JN, et al. Factors associated with the refusal of surgery and the associated impact on survival in patients with rectal cancer using the National Cancer Database. J Gastrointest Oncol. 2021;12(4):1482–97. https://doi.org/10.21037/jgo-20-437.
Moya JJ, Moazzez A, Ozao-Choy JJ, Dauphine C. Patients with invasive breast cancer who refuse treatment: an analysis of associated factors and impact on survival. Am Surg. 2021;87(10):1627–32. https://doi.org/10.1177/00031348211024170.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
RH and HK came up with the idea; RH, HK, WD, and FA were responsible for the metholdology; HK wrote the original draft; HK, EP, FA, WD, ME, LO, LR, JA, and RH were responsible for review and editing; and RH was responsible for supervision. All authors read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Disclosures
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was exempt from institutional review by the University Hospitals Cleveland Medical Center IRB committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kakish, H.H., Ahmed, F.A., Pei, E. et al. Understanding Factors Leading to Surgical Attrition for “Resectable” Gastric Cancer. Ann Surg Oncol 30, 4207–4216 (2023). https://doi.org/10.1245/s10434-023-13469-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-13469-5