Abstract
Background
Additional risk-stratification measures are needed in breast cancer patients with residual disease after neoadjuvant chemotherapy (NAC). We aimed to describe oncologic outcomes in a modern cohort treated with NAC, and evaluate the prognostic value of histologic pattern of residual tumor.
Patients and Methods
We included patients with stage I–III breast cancer treated with NAC and surgery from 2004 to 2014. Histologic pattern of residual tumor was evaluated by central pathology review when slides were available. Multivariable Cox regression was performed to evaluate factors associated with locoregional recurrence (LRR), recurrence-free survival (RFS), and overall survival (OS).
Results
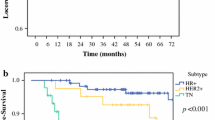
Among 975 patients, median follow-up was 74.0 months and 10-year rates of LRR, RFS, and OS were 9.8%, 67.6% and 74.4%, respectively. Biologic subtype, pathologic node-positive disease, and pathologic complete response (pCR) were associated with outcomes. Among 666 (68.3%) patients with central pathology review, pattern of residual disease was not significantly associated with LRR. However, both scattered residual tumor and no/minimal response relative to a concentric pattern of response were significantly associated with inferior RFS (scattered: hazard ratio 2.0, p = 0.015; no/minimal response: hazard ratio 2.2, p = 0.021) and OS (scattered: hazard ratio 2.2, p = 0.026; no/minimal response: hazard ratio 2.5, p = 0.023). This finding was most prominent in patients with triple-negative breast cancer.
Conclusions
Patients with a scattered relative to concentric pattern of residual tumor after NAC had inferior RFS and OS, nearly as poor as those with no/minimal response. Histologic pattern of residual tumor may represent a novel prognostic measure, particularly in the triple-negative breast cancer population.


Similar content being viewed by others
References
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72.
Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–59.
von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–28.
Mamounas EP, Anderson SJ, Dignam JJ, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol. 2012;30(32):3960–6.
Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12(5):320–7.
Penault-Llorca F, Abrial C, Raoelfils I, et al. Comparison of the prognostic significance of Chevallier and Sataloff’s pathologic classifications after neoadjuvant chemotherapy of operable breast cancer. Hum Pathol. 2008;39(8):1221–8.
Symmans WF, Wei C, Gould R, et al. Long-Term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35(10):1049–60.
Muller HD, Posch F, Suppan C, et al. Validation of residual cancer burden as prognostic factor for breast cancer patients after neoadjuvant therapy. Ann Surg Oncol. 2019;26(13):4274–83.
Mittendorf EA, Vila J, Tucker SL, et al. The Neo-Bioscore update for staging breast cancer treated with neoadjuvant chemotherapy: incorporation of prognostic biologic factors into staging after treatment. JAMA Oncol. 2016;2(7):929–36.
Yi M, Lin H, Bedrosian I, et al. Staging for breast cancer patients receiving neoadjuvant chemotherapy: utility of incorporating biologic factors. Ann Surg Oncol. 2020;27(2):359–66.
Kantor O, Bao J, Jaskowiak N, Yao K, Tseng J. The prognostic value of the AJCC 8th edition staging system for patients undergoing neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. 2020;27(2):352–8.
Mukhtar RA, Yau C, Rosen M, et al. Clinically meaningful tumor reduction rates vary by prechemotherapy MRI phenotype and tumor subtype in the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Ann Surg Oncol. 2013;20(12):3823–30.
Goorts B, Dreuning KMA, Houwers JB, et al. MRI-based response patterns during neoadjuvant chemotherapy can predict pathological (complete) response in patients with breast cancer. Breast Cancer Res. 2018;20(1):34.
Fukada I, Araki K, Kobayashi K, et al. Pattern of tumor shrinkage during neoadjuvant chemotherapy is associated with prognosis in low-grade luminal early breast cancer. Radiology. 2018;286(1):49–57.
Pastorello RG, Laws A, Grossmith S, et al. Clinico-pathologic predictors of patterns of residual disease following neoadjuvant chemotherapy for breast cancer. Mod Pathol. 2021;34(5):875–82.
Ferrari P, Scatena C, Ghilli M, Bargagna I, Lorenzini G, Nicolini A. Molecular mechanisms, biomarkers and emerging therapies for chemotherapy resistant TNBC. Int J Mol Sci. 2022;23(3):1665.
Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21.
Schmid P CJ, Dent R, et al. KEYNOTE-522: phase 3 study of neoadjuvant pembrolizumab plus chemotherapy versus placebo plus chemotherapy, followed by adjuvant pembrolizumab versus placebo for early stage triple-negative breast cancer. ESMO Virtual Plenary; 2021;2021.
Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol. 2004;22(12):2303–12.
Early Breast Cancer Trialists' Collaborative G. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39.
Boughey JC, Peintinger F, Meric-Bernstam F, et al. Impact of preoperative versus postoperative chemotherapy on the extent and number of surgical procedures in patients treated in randomized clinical trials for breast cancer. Ann Surg. 2006;244(3):464–70.
Choi J, Laws A, Hu J, Barry W, Golshan M, King T. Margins in breast-conserving surgery after neoadjuvant therapy. Ann Surg Oncol. 2018;25(12):3541–7.
Wimmer K, Bolliger M, Bago-Horvath Z, et al. Impact of surgical margins in breast cancer after preoperative systemic chemotherapy on local recurrence and survival. Ann Surg Oncol. 2020;27(5):1700–7.
Ling DC, Sutera PA, Iarrobino NA, et al. Is multifocal regression a risk factor for ipsilateral breast tumor recurrence in the modern era after neoadjuvant chemotherapy and breast conservation therapy? Int J Radiat Oncol Biol Phys. 2019;104(4):869–76.
Funding
Primary funding was provided by the Rob and Karen Hale Distinguished Chair in Surgical Oncology (to E.A.M.). Additional funding for the project was provided by the Pam Gelsomini Fellowship Fund (to A.L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
T.A.K. reports speakers honoraria and advisory board participation for Exact Sciences (formerly Genomic Health) and global advisory board participation for Besins Healthcare. E.A.M. reports clinical trial funding from Roche/Genentech (via SU2C grant) and Gilead; compensated advisory board service for, and consulting fees from, Roche/Genentech, Merck, and Exact Sciences; compensated advisory board service for AstraZeneca; honoraria from Physicians’ Education Resource; and uncompensated service on the Board of Directors for the American Society of Clinical Oncology, the steering committees of Roche/Genentech, Bristol-Myers Squibb, and Eli Lilly, and uncompensated service as a Scientific Advisor for Susan G. Komen for the Cure Foundation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior presentation: A subset of this data was presented as a poster presentation at the ASCO Annual Meeting 2020: Laws A., Pastorello R.G., Choi J., Kantor O., Grossmith S., Schnitt S.J., Golshan M., Mittendorf E.A., King T.A. The impact of pattern of tumor response and other post-treatment histologic features on local recurrence in patients treated with neoadjuvant chemotherapy and breast conservation. J Clin Oncol. 2020;.38:15_suppl:581. doi: 10.1200/JCO.2020.38.15_suppl.581.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Laws, A., Pastorello, R., Dey, T. et al. Impact of the Histologic Pattern of Residual Tumor After Neoadjuvant Chemotherapy on Recurrence and Survival in Stage I–III Breast Cancer. Ann Surg Oncol 29, 7726–7736 (2022). https://doi.org/10.1245/s10434-022-12054-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12054-6