Abstract
Background
Nodal-skip metastasis (NSM) is found in esophageal squamous cell carcinoma (ESCC), but its prognostic role is controversial. This study aimed to investigate the prognostic value of NSM for thoracic ESCC patients.
Methods
Categorization of NSM was according to the N groupings of Japan Esophagus Society (JES) staging system, which is dependent on tumor location. Using the Kaplan–Meier method and Cox-regression analysis, this study retrospectively analyzed the overall survival (OS) for 2325 ESCC patients after radical esophagectomy at three high-volume esophageal cancer centers. Predictive models also were constructed.
Results
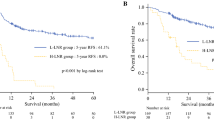
The overall NSM rate was 20% (229/1141): 37.4% in the in upper, 12.9% in the middle, and 22.2% in the lower thoracic ESCC. The patients with NSM always had a better prognosis than those without NSM. Furthermore, NSM was an independent prognostic factor for thoracic ESCC patients (hazard ratio [HR], 0.633; 95% confidence interval [CI], 0.499–0.803; P < 0.001). By integrating the prognostic values of NSM and N stage, the authors proposed the new N staging system. The categories defined by the new N staging system were more homogeneous in terms of OS than those defined by the current N system. Moreover, the new N system was shown to be an independent prognostic factor also for thoracic ESCC patients (HR, 1.607; 95% CI, 1.520–1.700; P < 0.001). Overall, the new N system had slightly better homogeneity, discriminatory ability, and monotonicity of gradient than the current N system.
Conclusions
This study emphasized the prognostic power of NSM and developed a modified node-staging system to improve the efficiency of the current International Union for Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) N staging system.


Similar content being viewed by others
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Global Burden of Disease Cancer Collaboration. The global burden of cancer 2013. JAMA Oncol. 2015;1:505–27.
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods, and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381–7.
Schaafsma T, Wakefield J, Hanisch R, et al. Africa’s oesophageal cancer corridor: geographic variations in incidence correlate with certain micronutrient deficiencies. PLoS ONE. 2015;10:e0140107.
Rafiemanesh H, Maleki F, Mohammadian-Hafshejani A, et al. The trend in histological changes and the incidence of esophagus cancer in Iran (2003–2008). Int J Prev Med. 2016;7:31.
Rice T.W, Ishwaran H, Hofstetter WL, et al. Esophageal cancer: associations with (pN +) lymph node metastases. Ann Surg. 2017;265:122–9.
Gertler R, Stein HJ, Schuster T, et al. Prevalence and topography of lymph node metastases in early esophageal and gastric cancer. Ann Surg. 2014;259:96–101.
Japanese Classification of Esophageal Cancer, 11th ed. Part I. Esophagus. 2017;14:1–36.
Amin MB, Edge S, Greene FL, et al. AJCC Cancer Staging Manual (M). 8th ed. Springer, New York, 2017. pp 185–202.
Zheng YZ, Zhao W, Hu, Y, et al. Aggressive surgical resection does not improve survival in operable esophageal squamous cell carcinoma with N2–3 status. World J Gastroenterol. 2015;21:8644–52.
Yuan Y, Hong HG, Zeng X, et al. Lymph node station-based nodal-staging system for esophageal squamous cell carcinoma: a large-scale multicenter study. Ann Surg Oncol. 2019;26:4045–52.
Xu QR, Zhuge XP, Zhang HL, et al. The N-classification for esophageal cancer staging: should it be based on number, distance, or extent of the lymph node metastasis? World J Surg. 2011;35:1303–10.
Chen SB, Weng HR, Wang G, et al. Prognostic factors and outcome for patients with esophageal squamous cell carcinoma underwent surgical resection alone: evaluation of the seventh edition of the American Joint Committee on Cancer staging system for esophageal squamous cell carcinoma. J Thorac Oncol. 2013;8:495–501.
Wu J, Chen QX, Zhou XM, et al. Prognostic significance of solitary lymph node metastasis in patients with squamous cell carcinoma of middle thoracic esophagus. World J Surg Oncol. 2012;10:210.
Prenzel KL, Bollschweiler E, Schroder W, et al. Prognostic relevance of skip metastases in esophageal cancer. Ann Thorac Surg. 2010;90:1662–7.
Prenzel KL, Monig SP, Sinning JM, et al. Role of skip metastasis to mediastinal lymph nodes in non-small cell lung cancer. J Surg Oncol. 2003;82:256–60.
Machens AH, Holzhausen J, Dralle H. Skip metastases in thyroid cancer leaping the central lymph node compartment. Arch Surg. 2004;139:43–5.
Chen J, Liu S, Pan J, et al. The pattern and prevalence of lymphatic spread in thoracic oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg. 2009;36:480–6.
Castoro C, Scarpa M, Cagol M, et al. Nodal metastasis from locally advanced esophageal cancer: how neoadjuvant therapy modifies their frequency and distribution. Ann Surg Oncol. 2011;18:3743–54.
Kayani B, Zacharakis E, Ahmed K, et al. Lymph node metastases and prognosis in oesophageal carcinoma: a systematic review. Eur J Surg Oncol. 2011;37:747–53.
Akutsu Y, Shuto K, Kono T, et al. The number of pathologic lymph nodes involved is still a significant prognostic factor even after neoadjuvant chemoradiotherapy in esophageal squamous cell carcinoma. J Surg Oncol. 2012;105:756–60.
Walther B, Johansson J, Johnsson F, et al., Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction: a prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg. 2003;238:803–12; discussion 812–4.
Urschel JD, Blewett CJ, Bennett WF, et al. Handsewn or stapled esophagogastric anastomoses after esophagectomy for cancer: meta-analysis of randomized controlled trials. Dis Esophagus. 2001;14:212–7.
Qing JJ, Li Y. Introduction to the TNM staging of esophageal cancer in the 11th edition of the Japan Esophageal Association (JES). Chinese J Clin Thorac Cardiovasc Surg. 2017;24:657–60
Cox DR. Regression models and life-tables (with discussion). Series B: methodology. J R Statist Soc. 1972;34:187–220.
Chen J, Chen C, He Y, Wu K, Wu H, Cai S. A new pN staging system based on both the number and anatomic location of metastatic lymph nodes in gastric cancer. G Gastrointest Surg. 2014;8:2080–8.
Nafteux P, Lerut T, De Hertogh G, et al. Can extracapsular lymph node involvement be a tool to fine-tune pN1 for adenocarcinoma of the oesophagus and gastro-oesophageal junction in the Union Internationale Contre le Cancer (UICC) TNM 7th edition. Eur J Cardiothorac Surg. 2014;45:1001–10.
Kuwano H, Nishimura Y, Oyama T, et al. Guidelines for Diagnosis and treatment of carcinoma of the esophagus, April 2012, edited by the Japan Esophageal Society. Esophagus. 2015;12:1–30.
He SL, Yang YS, Wang WP, et al. Prognostic evaluation of nodal skip metastasis for thoracic esophageal squamous cell carcinoma. Ann Thorac Surg. 2019;108:1717–23.
Kumakura Y, Yokobori T, Yoshida T, et al. Elucidation of the anatomical mechanism of nodal skip metastasis in superficial thoracic esophageal squamous cell carcinoma. Ann Surg Oncol. 2017;25:1221–8.
Tachimori Y, Nagai Y, Kanamori N, Hokamura N, Lgaki H. Pattern of lymph node metastases of esophageal squamous cell carcinoma based on the anatomical lymphatic drainage system. Dis Esophagus. 2011;24:33–8.
Yang YS, Hu WP, Wang WP, Yuan Y. Nodal skip metastasis may undermine the predictive power of topographic pN classification in esophageal squamous cell carcinoma. Surgery. 2018;21:17.
Wang F, Zheng YZ, Wang Z, Zheng QF. Nodal skip metastasis in esophageal squamous cell carcinoma patients undergoing three-field lymphadenectomy. Ann Thorac Surg. 2017;104:1187–93.
Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8.
Long L, Rubin R, Brodt P. Enhanced invasion and liver colonization by lung carcinoma cells overexpressing the type 1 insulin-like growth factor receptor. Exp Cell Res. 1998;238:116–21.
Yekebas EF Schurr PG, Kaifi JT, et al. Effectiveness of radical en bloc esophagectomy compared to transhiatal esophagectomy in squamous cell cancer of the esophagus is influenced by nodal micrometastases. J Surg Oncol. 2006;93:541–9.
Izbicki JR, Hosch SB, Pichlmeier U, et al. Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl J Med. 1997;337:1188–94.
Hosch SB, Stoecklein NH, Pichlmeier U, et al. Esophageal cancer: the mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol. 2001;19:1970–5.
Acknowledgments
The authors thank the Department of Pathology of West China Hospital, Shantou University Medical College, and Sun Yat-sen University Cancer Centre for their substantial work in detecting and analyzing the blood samples.
Author information
Authors and Affiliations
Contributions
(I) Conception and design: Long-Qi Chen, Yin Li, Zhi-Yong Wu, Jian-Hua Fu, Xiu-E Xu, Jian-Yi Wu, Yu-Shang Yang and Qi-Xin Shang; (II) Administrative support: Li-Yan Xu, Hong Yang; (III) Provision of study materials or patients: Li-Yan Xu, Yin Li, Zhi-Yong Wu, Jian-Hua Fu, Jian-Yi Wu, Long-Qi Chen; (IV) Collection and assembly of data: Qi-Xin Shang, Yu-Shang Yang, Xiu-E Xu, Jian-Yi Wu; (V) Figure preparation: Qi-Xin Shang. (VI) Data analysis and interpretation: Qi-Xin Shang and Long-Qi Chen; (VII) Statistic review: Zhi-Wei Fan, Yong Yuan. (VIII) Manuscript writing: All authors; (IX) Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Disclosure
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Fig. S1
Kaplan-Meier survival plot by nodal-skip metastasis (NSM) group stratification for patients in different UICC/AJCC N stages. N1 with NSM vs N1 without NSM (P = 0.849); N1 with NSM vs N2 with NSM (P = 0.783); N1 with NSM vs N3 with NSM (P = 0.665); N1 without NSM vs N2 with NSM (P = 0.768); N1 without NSM vs N3 with NSM (P = 0.643); N1 with NSM vs N2 without NSM (P < 0.001); N1 with NSM vs N3 without NSM (P < 0.001); N2 with NSM vs N2 without NSM (P = 0.017); N3 with NSM vs N3 without NSM (P = 0.025). Censored: The number of patients who did not die at the end of the follow-up period. (TIFF 220 kb)
Fig. S2
Average survival time by nodal-skip metastasis (NSM) group stratification for the patients in different UICC/AJCC N stages of disease. The average survival times for the patients who had N0 stage, N1 stage with NSM, N2 stage with NSM, and N3 stage with NSM were respectively 76.737 ± 1.561, 51.005 ± 3.204, 49.607 ± 6.809, and 49.662 ± 1.561 months, whereas the average survival time for the patients who had N1, N2 and N3 stage without NSM were respectively 49.756 ± 2.105, 32.620 ± 1.749, and 21,665 ± 1.755 months. (TIFF 38 kb)
Fig. S3
The cut-off point of age. 55 is regarded as the optimal cut-off point for age in this study after X-tile analysis. (TIFF 244 kb)
Rights and permissions
About this article
Cite this article
Shang, QX., Yang, YS., Xu, LY. et al. Prognostic Role of Nodal Skip Metastasis in Thoracic Esophageal Squamous Cell Carcinoma: A Large-Scale Multicenter Study. Ann Surg Oncol 28, 6341–6352 (2021). https://doi.org/10.1245/s10434-020-09509-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-09509-z