Abstract
Background
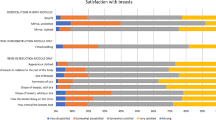
The BREAST-Q is a patient-reported outcome measure to evaluate satisfaction and health-related quality of life (HRQOL) after breast surgery. The aim of this study is to test the acceptability, reliability, and validity of the most recently developed BREAST-Q module for breast-conserving therapy (BCT) in a prospective clinical cohort.
Methods
The BREAST-Q BCT module was translated into German according to international guidelines. A total of 253 women with primary breast cancer undergoing BCT were recruited preoperatively. This study evaluated the BREAST-Q BCT subscales by using psychometric methods including acceptability, reliability, and validity. To examine construct validity, convergent and discriminant validity were determined by testing the instrument’s scales against the EORTC C30 and BR23 as reference questionnaires.
Results
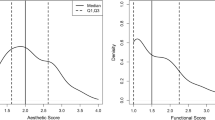
Acceptability was supported by a high follow-up rate (90%) and low frequency of missing data (< 10%) in all but three scales. Scale reliability was supported by high Cronbach’s alpha coefficients (> 0.86) and item-total correlations (range of means, 0.33–0.89). Validity was shown by convergent and divergent correlations. The hypotheses of relationships between the scales of the BREAST-Q and the EORTC QLQ C30 BR23 revealed moderate to high correlations.
Conclusions
The BREAST-Q BCT module proved to be an accepted, reliable, and valid questionnaire for the assessment of HRQOL and patient satisfaction after BCT in breast cancer patients. It can be recommended as a possible standard PROM for individual clinical analysis, quality assessment, and future trials.
Similar content being viewed by others
References
Ferlay J, Héry C, Autier P, Sankaranarayanan R. Global burden of breast cancer. Breast Cancer Epidemiol: Springer; 2010:1–19.
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. Mar 1 2015;136(5):E359–386.
Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res 2008;27(1):1.
Nicholson RM, Leinster S, Sassoon EM. A comparison of the cosmetic and psychological outcome of breast reconstruction, breast conserving surgery and mastectomy without reconstruction. Breast. 2007;16(4):396–410.
Curran D, Van Dongen J, Aaronson N, et al. Quality of life of early-stage breast cancer patients treated with radical mastectomy or breast-conserving procedures: results of EORTC trial 10801. Eur J Cancer. 1998;34(3):307–314.
Krishnan L, Stanton AL, Collins CA, Liston VE, Jewell WR 2001 Form or function? Part 2: Objective cosmetic and functional correlates of quality of life in women treated with breast-conserving surgical procedures and radiotherapy. Cancer 91(12):2282–2287.
Nano MT, Gill PG, Kollias J, Bochner MA, Malycha P, Winefield HR. Psychological impact and cosmetic outcome of surgical breast cancer strategies. ANZ J Surg. 2005;75(11):940–947.
Wang HT, Barone CM, Steigelman MB, et al. Aesthetic outcomes in breast conservation therapy. Aesthetic Surg J. 2008;28(2):165–170.
Reaby LL, Hort LK, Vandervord J. Body image, self-concept, and self-esteem in women who had a mastectomy and either wore an external breast prosthesis or had breast reconstruction and women who had not experienced mastectomy. Health Care Women Int. 1994;15(5):361–375.
Javid SH, Lawrence SO, Lavallee DC. Prioritizing patient-reported outcomes in breast cancer surgery quality improvement. Breast J. Mar 2017;23(2):127–137.
Van Der Wees PJ, Nijhuis Van Der Sanden MW, Ayanian JZ, Black N, Westert GP, Schneider EC (2014) Integrating the use of patient-reported outcomes for both clinical practice and performance measurement: views of experts from 3 countries. Milbank Q 92(4):754–775.
Pusic AL, Chen CM, Cano S, et al (2007) Measuring quality of life in cosmetic and reconstructive breast surgery: a systematic review of patient-reported outcomes instruments. Plast Reconstr Surg 120(4):823–837.
Noh DY, Nam SJ, Ahn SH, et al. Association of clinical experiences with patient-reported outcomes among breast cancer surgery patients: breast cancer quality care study. Qual Life Res. Mar 2008;17(2):215–225.
Boone WJ (2016) Rasch analysis for instrument development why when and how. CBE Life Sci Ed 15(4):4.
Fayers PM, Machin D. Quality of life: the assessment, analysis and interpretation of patient-reported outcomes. Wiley, New York, 2013.
Aaronson N, Alonso J, Burnam A, et al. Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res. May 2002;11(3):193–205.
Health UDo, Evaluation HSFCfD, Research, et al. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:1-20.
Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. Aug 2009;124(2):345–353.
O’Connell RL, DiMicco R, Khabra K, et al. Initial experience of the BREAST-Q breast-conserving therapy module. Breast Cancer Res Treat. Nov 2016;160(1):79–89.
McKenna SP, Heaney A, Wilburn J, Stenner AJ. Measurement of patient-reported outcomes. 1: The search for the Holy Grail. J Med Econ. 2019:1–7.
Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167.
Acquadro C, ed Linguistic validation manual for health outcome assessments. New ed. Lyon: Mapi Research Institute; 2012.
Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. JNCI: J Natl Cancer Inst. 1993;85(5):365–376.
Letellier ME, Dawes D, Mayo N. Content verification of the EORTC QLQ-C30/EORTC QLQ-BR23 with the International Classification of Functioning, Disability and Health. Qual Life Res. Mar 2015;24(3):757–768.
Michels FA, Latorre Mdo R, Maciel Mdo S. Validity, reliability and understanding of the EORTC-C30 and EORTC-BR23, quality of life questionnaires specific for breast cancer. Rev Bras Epidemiol. Jun 2013;16(2):352–363.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Erlbaum; 1988.
Jeevan R, Cromwell DA, Browne JP, et al. Findings of a national comparative audit of mastectomy and breast reconstruction surgery in England. J Plast Recon Aesthet Surg. 2014;67(10):1333–1344.
Waljee J, McGlinn EP, Sears ED, Chung KC. Patient expectations and patient-reported outcomes in surgery: a systematic review. Surgery. May 2014;155(5):799–808.
Heil J, Holl S, Golatta M, et al. Aesthetic and functional results after breast conserving surgery as correlates of quality of life measured by a German version of the Breast Cancer Treatment Outcome Scale (BCTOS). Breast. Dec 2010;19(6):470–474.
Al-Ghazal SK, Fallowfield L, Blamey R. Does cosmetic outcome from treatment of primary breast cancer influence psychosocial morbidity? Eur J Surg Oncol. 1999;25(6):571–573.
Reich M, Lesur A, Perdrizet-Chevallier C. Depression, quality of life and breast cancer: a review of the literature. Breast Cancer Res Treat. 2008;110(1):9–17.
Fingeret MC, Teo I, Epner DE. Managing body image difficulties of adult cancer patients: lessons from available research. Cancer. 2014;120(5):633–641.
Waljee JF, Hu ES, Ubel PA, Smith DM, Newman LA, Alderman AK. Effect of esthetic outcome after breast-conserving surgery on psychosocial functioning and quality of life. J Clin Oncol. 2008;26(20):3331–3337.
Gu J, Groot G, Boden C, Busch A, Holtslander L, Lim H. Review of factors influencing women’s choice of mastectomy versus breast conserving therapy in early stage breast cancer: a systematic review. Clin Breast Cancer. Jan 3 2018.
Efficace F, Bottomley A, Coens C, et al. Does a patient’s self-reported health-related quality of life predict survival beyond key biomedical data in advanced colorectal cancer? Eur J Cancer. 2006;42(1):42–49.
Gupta D, Rodeghier M, Lis CG. Patient satisfaction with service quality as a predictor of survival outcomes in breast cancer. Support Care Cancer. January 01 2014;22(1):129–134.
Cano SJ, Klassen AF, Scott AM, Cordeiro PG, Pusic AL. The BREAST-Q: further validation in independent clinical samples. Plast Reconstr Surg. Feb 2012;129(2):293–302.
Wright BD, Mok M. Understanding Rasch measurement: Rasch models overview. J Appl Measure. 2000.
Acknowledgment
We would like to thank Michael Hanna, Ph.D. (Mercury Medical Research and Writing) for proof-reading the manuscript and providing feedback on it, especially in regards to the high Cronbach’s alphas.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interests (e.g. employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding) by any of the authors with regard to this paper.
Ethical Approval
The study was approved by the ethics commission of the University of Heidelberg Medical School. The study was deemed to be without risk, including only anonymized analysis of routinely collected data; consequently the ethics committee of the University of Heidelberg did not request approval for consent for this analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stolpner, I., Heil, J., Feißt, M. et al. Clinical Validation of the BREAST-Q Breast-Conserving Therapy Module. Ann Surg Oncol 26, 2759–2767 (2019). https://doi.org/10.1245/s10434-019-07456-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07456-y