Abstract
Background
Increasing evidence suggests that postoperative infection is associated with poorer long-term outcome in various malignancies. However, the mechanism of poor prognosis induced by postoperative infection has not been clearly explained. We sought to determine whether abdominal infection promotes cancer metastases in a murine liver metastasis model, and to investigate the role of liver natural killer (NK) cells on antitumor immunity during abdominal infection.
Methods
Female BALB/c (8–10 weeks old) mice were inoculated with NL-17 colon cancer cells into the spleen and then subjected to abdominal infection induced by cecal ligation and puncture (CLP) or sham treatment. The extent of liver metastases and cytokine production in the serum and liver were investigated. Cell fraction and cytotoxic activities of liver mononuclear cells (MNCs) were elucidated.
Results
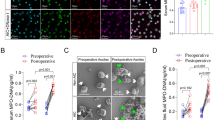
CLP mice had poorer survival and their serum levels of IL-6, -10, and -12p70 were significantly elevated on day 1 compared with sham-treated and control mice. No obvious differences in cytokine levels of the liver homogenates were identified among the three groups, except IL-12p70 levels in CLP mice on day 7 significantly decreased. The cytotoxic activities of liver MNCs were significantly suppressed in CLP mice soon after tumor inoculation. Flow cytometry revealed a decrease in NK cells in the liver and perforin and granzyme B expression levels.
Conclusions
Abdominal infection promoted liver metastases in a murine liver metastasis model, which may be partially caused by a decrease in the number and activity of NK cells during abdominal infection.





Similar content being viewed by others
References
Collins TC, Daley J, Henderson WH, Khuri SF. Risk factors for prolonged length of stay after major elective surgery. Ann Surg. 1999;230:251–9.
Tsujimoto H, Ichikura T, Ono S, et al. Impact of postoperative infection on long-term survival after potentially curative resection for gastric cancer. Ann Surg Oncol. 2009;16:311–8.
DerHagopian RP, Sugarbaker EV, Ketcham A. Inflammatory oncotaxis. JAMA. 1978;240:374–5.
de Melo GM, Ribeiro KC, Kowalski LP, Deheinzelin D. Risk factors for postoperative complications in oral cancer and their prognostic implications. Arch Otolaryngol Head Neck Surg. 2001;127:828–33.
Akyol AM, McGregor JR, Galloway DJ, Murray GD, George WD. Anastomotic leaks in colorectal cancer surgery: a risk factor for recurrence? Int J Colorectal Dis. 1991;6:179–83.
Fujita S, Teramoto T, Watanabe M, Kodaira S, Kitajima M. Anastomotic leakage after colorectal cancer surgery: a risk factor for recurrence and poor prognosis. Jpn J Clin Oncol. 1993;23:299–302.
Varty PP, Linehan IP, Boulos PB. Intra-abdominal sepsis and survival after surgery for colorectal cancer. Br J Surg. 1994;81:915–8.
Nespoli A, Gianotti L, Totis M, et al. Correlation between postoperative infections and long-term survival after colorectal resection for cancer. Tumori. 2004;90:485–90.
Walker KG, Bell SW, Rickard MJ, et al. Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg. 2004;240:255–9.
McArdle CS, McMillan DC, Hole DJ. Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg. 2005;92:1150–4.
Law WL, Lee YM, Choi HK, Seto CL, Ho JW. Impact of laparoscopic resection for colorectal cancer on operative outcomes and survival. Ann Surg. 2007;245:1–7.
Tsujimoto H, Ueno H, Hashiguchi Y, Ono S, Ichikura T, Hase K. Postoperative infections are associated with adverse outcome after resection with curative intent for colorectal cancer. Oncol Lett. 2010;1:119–25.
Hirai T, Yamashita Y, Mukaida H, Kuwahara M, Inoue H, Toge T. Poor prognosis in esophageal cancer patients with postoperative complications. Surg Today. 1998;28:576–9.
Rizk NP, Bach PB, Schrag D, et al. The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. J Am Coll Surg. 2004;198:42–50.
Lagarde SM, de Boer JD, ten Kate FJ, Busch OR, Obertop H, van Lanschot JJ. Postoperative complications after esophagectomy for adenocarcinoma of the esophagus are related to timing of death due to recurrence. Ann Surg. 2008;247:71–6.
Ito H, Are C, Gonen M, et al. Effect of postoperative morbidity on long-term survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247:994–1002.
Sierzega M, Kolodziejczyk P, Kulig J; Polish Gastric Cancer Study Group. Impact of anastomotic leakage on long-term survival after total gastrectomy for carcinoma of the stomach. Br J Surg. 2010;97:1035–42.
Tsujimoto H, Ono S, Mochizuki H, et al. Role of macrophage inflammatory protein 2 in acute lung injury in murine peritonitis. J Surg Res. 2002;103:61–7.
Tsujimoto H, Ono S, Matsumoto A, et al. A critical role of CpG motifs in a murine peritonitis model by their binding to highly expressed Toll-like receptor-9 on liver NKT cells. J Hepatol. 2006;45:836–43.
Neri S, Mariani E, Meneghetti A, Cattini L, Facchini A. Calcein–acetyoxymethyl cytotoxicity assay: standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin Diagn Lab Immunol. 2001;8:1131–5.
Cools-Lartigue J, Spicer J, McDonald B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446–58.
Efron P, Moldawer LL. Sepsis and the dendritic cell. Shock. 2003;20:386–401.
Ashizawa T, Okada R, Suzuki Y, et al. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: role of IL-6 as a prognostic factor. Gastric Cancer. 2005;8:124–31.
Gately MK, Renzetti LM, Magram J, et al. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Ann Rev Immunol. 1998;16:495–521.
Matsumoto A, Tsujimoto H, Ono S, et al. Loss of hepatic B cells following lipopolysaccharide injection and polymicrobial sepsis. J Gastroenterol Hepatol. 2009;24:262–9.
Frese-Schaper M, Keil A, Yagita H, et al. Influence of natural killer cells and perforinmediated cytolysis on the development of chemically induced lung cancer in A/J mice. Cancer Immunol Immunother. 2014;63:571–80.
Jaime-Ramirez AC, Mundy-Bosse BL, Kondadasula S, et al. IL-12 enhances the antitumor actions of trastuzumab via NK cell IFN-gamma production. J Immunol. 2011;186:3401–9.
Indrova M, Simova J, Bieblova J, Bubenik J, Reinis M. NK1.1+ cells are important for the development of protective immunity against MHC I-deficient, HPV16-associated tumours. Oncol Rep. 2011;25:281–8.
Acknowledgment
The authors are grateful to Kouji Matsumura (Central Research Laboratory, National Defense Medical College, Saitama, Japan) for his technical support concerning cell culture.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2015_4466_MOESM1_ESM.pdf
Analyses of cytokine levels in the liver homogenates in CLP and sham mice without tumor inoculation. There were no differences of cytokine levels in the liver homogenates in CLP and sham mice without tumor inoculation (n=4). Each experiment was independently performed 3 times with similar results. Representative data are depicted.White bar = sham mice, black bars = CLP mice (PDF 29 kb)
Rights and permissions
About this article
Cite this article
Matsumoto, Y., Tsujimoto, H., Ono, S. et al. Abdominal Infection Suppresses the Number and Activity of Intrahepatic Natural Killer Cells and Promotes Tumor Growth in a Murine Liver Metastasis Model. Ann Surg Oncol 23 (Suppl 2), 257–265 (2016). https://doi.org/10.1245/s10434-015-4466-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4466-7