Abstract
Background
Deregulation of apoptosis will influence the balance of cell proliferation and cell death, resulting in various fatal diseases that can include cancer. In prior research reports related to cancer therapy, phytohemagglutinin, a lectin extracted from red kidney beans, demonstrated the ability to inhibit the growth of human cancer cells. However, one of its isoforms, erythroagglutinating, has yet to be evaluated on its anticancer effects.
Methods
PHA-E was used to induce apoptosis of A-549 lung cancer cells and the possible signal transduction pathway was elucidated, as measured by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, G6PD release assay, flow cytometry, and Western blot analysis.
Results
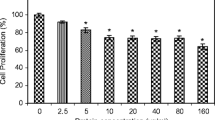
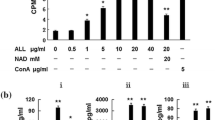
PHA-E treatment caused a dose-dependent increase of cell growth inhibition and cytotoxicity on A-549 cells. In annexin V/propidium iodide [i.e., PI] and TUNEL (terminal deoxynucleotidyl transferase dUTP nick-end labeling)/PI assay, we found that the rate of apoptotic cells was raised as the concentration of PHA-E increased. Treatment of A-549 cells with PHA-E resulted in enhancing the release of cytochrome c, which thus activated an increase in caspase 9 and caspase 3, the upregulation of Bax and Bad, the downregulation of Bcl-2 and phosphorylated Bad, and finally the inhibition of the epidermal growth factor receptor and its downstream signal pathway PI3K/Akt and MEK/ERK.
Conclusions
PHA-E can induce growth inhibition and cytotoxicity of lung cancer cells, which is mediated through an activation of the mitochondria apoptosis pathway. These results suggest that PHA-E can be developed into a new therapeutic treatment that can be applied as an effective anti–lung cancer drug in the near future.






Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18:1059–68.
Hu W, Kavanagh JJ. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. 2003;4:721–9.
Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57.
Lockshin RA, Zakeri Z. Programmed cell death and apoptosis: origins of the theory. Nat Rev Mol Cell Biol. 2001;2:545–50.
Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12.
Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–90.
Strasser A, O’Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–45.
Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–77.
Ozören N, El-Deiry WS. Cell surface death receptor signaling in normal and cancer cells. Semin Cancer Biol. 2003;13:135–47.
Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37.
Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59.
Zornig M, Hueber A, Baum W, Evan G. Apoptosis regulators and their role in tumorigenesis. Biochim Biophys Acta. 2001;1551:F1–37.
Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–19.
Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–85.
Khan N, Afaq F, Mukhtar H. Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis. 2007;28:233–9.
De Mejía EG, Prisecaru VI. Lectins as bioactive plant proteins: a potential in cancer treatment. Crit Rev Food Sci Nutr. 2005;45:425–45.
Leavitt RD, Feldsted RL, Bachur NR. Biological and biochemical properties of Phaseolus vulgaris. isolectins. J Biol Chem. 1977;252:2961–6.
Rüdiger H, Gabius HJ. Plant lectins: occurrence, biochemistry, functions and applications. Glycoconj J. 2001;18:589–613.
Zhang JS, Shi J, Ilic S, Xue SJ, Kakuda Y. Biological properties and characterization of lectin from red kidney bean (Phaseolus vulgaris.). Food Rev Int. 2009;25:12–27.
O’Flynn K, Krensky AM, Beverley PC, Burakoff SJ, Linch DC. Phytohaemagglutinin activation of T cells through the sheep red blood cell receptor. Nature. 1985;313:686–7.
Yashwantrai NV, Flossie WS. The biochemistry of AIDS. Annu Rev Biochem. 1991;60:577–630.
Ye XY, Ng TB, Tsang WK, Wang J. Isolation of a homodimeric lectin with antifungal and antiviral activities from red kidney bean (Phaseolus vulgaris.) seeds. J Protein Chem. 2001;20:367–75.
Kiss R, Camby I, Duckworth C, et al. In vitro influence of Phaseolus vulgaris, Griffonia simplicifolia,. concanavalin A, wheat germ, and peanut agglutinins on HCT-15, LoVo, and SW837 human colorectal cancer cell growth. Gut. 1997;40:253–61.
De Mejía EG, Prisecaru VI. Lectins as bioactive plant proteins: a potential in cancer treatment. Crit Rev Food Sci Nutr. 2005;45:425–45.
Wimer BM. Therapeutic activities of PHA-L4, the mitogenic isolectin of phytohemagglutinin. Mol Biother. 1990;2:74–90.
Nyati MK, Morgan MA, Feng FY, Lawrence TS. Integration of EGFR inhibitors with radiochemotherapy. Nat Rev Cancer. 2006;6:876–85.
Hickman JA. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992;11:121–39.
Handerson T, Camp R, Harigopal M, Rimm D, Pawelek J. Beta1,6-branched oligosaccharides are increased in lymph node metastases and predict poor outcome in breast carcinoma. Clin Cancer Res. 2005;11:2969–73.
Rebbaa A, Chou PM, Vucic I, et al. Expression of bisecting GlcNAc in pediatric brain tumors and its association with tumor cell response to vinblastine. Clin Cancer Res. 1999;5:3661–8.
Von Ahsen O, Waterhouse NJ, Kuwana T, Newmeyer DD, Green DR. The “harmless” release of cytochrome c.. Cell Death Differ. 2000;7:1192–9.
Sharpe JC, Arnoult D, Youle RJ. Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta. 2004;1644:107–13.
Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56.
Fletcher JI, Meusburger S, Hawkins CJ, et al. Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc Natl Acad Sci USA. 2008;105:18081–7.
Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell. 1996;87:619–28.
Chen L, Willis SN, Wei A, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403.
Willis SN, Fletcher JI, Kaufmann T, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–9.
Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41.
Datta SR, Ranger AM, Lin MZ, et al. Survival factor–mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev Cell. 2002;3:631–43.
Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci. 2008;65:1566–84.
Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12:5268–72.
Lacroix L, Besse B, Bidart JM, Bosq J. KRAS status versus EGFR status in lung cancer therapy. Bull Cancer. 2009;96:S75–83.
Mukohara T, Kudoh S, Yamauchi S, et al. Expression of epidermal growth factor receptor (EGFR) and downstream-activated peptides in surgically excised non-small-cell lung cancer (NSCLC). Lung Cancer. 2003;41:123–30.
Tanno S, Ohsaki Y, Nakanishi K, Toyoshima E, Kikuchi K. Small cell lung cancer cells express EGFR and tyrosine phosphorylation of EGFR is inhibited by gefitinib (“Iressa,” ZD1839). Oncol Rep. 2004;12:1053–7.
Acknowledgment
We thank the China Medical University, for financially supporting this research under contract CMU97-230.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
C.-H. Yao, Y.-S. Chen and G.-C. Dong contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kuo, WT., Ho, YJ., Kuo, SM. et al. Induction of the Mitochondria Apoptosis Pathway by Phytohemagglutinin Erythroagglutinating in Human Lung Cancer Cells. Ann Surg Oncol 18, 848–856 (2011). https://doi.org/10.1245/s10434-010-1351-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-1351-2