Abstract
Background
Nodal metastasis is considered a major prognostic factor in patients with ampulla of Vater carcinoma (AVC). No study has investigated the significance of the ratio between metastatic and resected/examined lymph nodes (LNR) in patients with AVC.
Methods
Demographic, operative, and pathology data, including number of resected/evaluated nodes and LNR, were collected from patients who underwent pancreaticoduodenectomy with radical intent for invasive AVC from 1990 to 2005. Survival rates and recurrence patterns were evaluated and predictors were identified.
Results
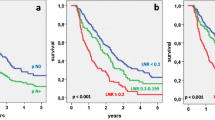
In 90 evaluable patients (51 males, 39 females, median age 62.5 years), 5-year disease-specific survival (DSS) was 61%. The median number of resected/evaluated nodes was 16 (range: 5–47); 50% of the patients had nodal metastases. The 5-year DSS according to LNR was 75%, 49%, 38%, and 0% for LNR = 0, LNR >0 and ≤0.2, LNR >0.2, and ≤0.4, and LNR >0.4 (P = 0.002), respectively. The 5-year DSS was 81% in patients with >16 resected/evaluated nodes compared with 45% in those with ≤16 resected/evaluated nodes (P = 0.001). On multivariate analysis LNR and a number of resected/evaluated nodes >16 were significant predictors of survival; a number of resected/evaluated nodes >16 was also the only independent predictor of recurrence.
Conclusions
After curative resection for AVC, LNR and a cutoff of 16 resected/evaluated nodes are powerful prognostic factors. LNR might represent a major parameter for patient stratification in adjuvant treatment trials.



Similar content being viewed by others
References
Duffy JP, Hines OJ, Liu JH, et al. Improved survival for adenocarcinoma of the ampulla of Vater: fifty-five consecutive resections. Arch Surg 2003; 138:941–8; discussion 948–50
Talamini MA, Moesinger RC, Pitt HA, et al. Adenocarcinoma of the ampulla of Vater. A 28-year experience. Ann Surg 1997; 225:590–9; discussion 599–600
Howe JR, Klimstra DS, Moccia RD, et al. Factors predictive of survival in ampullary carcinoma. Ann Surg 1998; 228:87–94
Riall TS, Cameron JL, Lillemoe KD, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-yearfollow-up. Surgery 2006; 140:764–72
Brown KM, Tompkins AJ, Yong S, et al. Pancreaticoduodenectomy is curative in the majority of patients with node-negative ampullary cancer. Arch Surg 2005; 140:529–32; discussion 532–33
Qiao QL, Zhao YG, Ye ML, et al. Carcinoma of the ampulla of Vater: factors influencing long-term survival of 127 patients with resection. World J Surg 2007; 31:137–43; discussion 144–6
Kim RD, Kundhal PS, McGilvray ID, et al. Predictors of failure after pancreaticoduodenectomy for ampullary carcinoma. J Am Coll Surg 2006; 202:112–9
Iacono C, Verlato G, Zamboni G, et al. Adenocarcinoma of the ampulla of Vater: T-stage, chromosome 17p allelic loss, and extended pancreaticoduodenectomy are relevant prognostic factors. J Gastrointest Surg 2007; 11:578–8
Park JS, Yoon DS, Kim KS, et al. Factors influencing recurrence after curative resection for ampulla of Vater carcinoma. J Surg Oncol 2007; 95:286–90
Hsu HP, Yang TM, Hsieh YH, et al. Predictors for patterns of failure after pancreaticoduodenectomy in ampullary cancer. Ann Surg Oncol 2007; 14:50–60
Roggin KK, Yeh JJ, Ferrone CR, et al. Limitations of ampullectomy in the treatment of nonfamilial ampullary neoplasms. Ann Surg Oncol 2005; 12:971–80
Todoroki T, Koike N, Morishita Y, et al. Patterns and predictors of failure after curative resections of carcinoma of the ampulla of Vater. Ann Surg Oncol 2003;10:1176–83
Yoon YS, Kim SW, Park SJ, et al. Clinicopathologic analysis of early ampullary cancers with a focus on the feasibility of ampullectomy. Ann Surg 2005; 242:92–100
Beger HG, Treitschke F, Gansauge F, et al. Tumor of the ampulla of Vater: experience with local or radical resection in 171 consecutively treated patients. Arch Surg 1999; 134:526–32
Bettschart V, Rahman MQ, Engelken FJ, et al. Presentation, treatment and outcome in patients with ampullary tumours. Br J Surg 2004; 91:1600–7
Sakata E, Shirai Y, Yokoyama N, et al. Clinical significance of lymph node micrometastasis in ampullary carcinoma. World J Surg 2006; 30:985–91
Sakata J, Shirai Y, Wakai T, et al. Number of positive lymph nodes independently affects long-term survival after resection in patients with ampullary carcinoma. Eur J Surg Oncol 2007; 33:346–51
Maithel SK, Khalili K, Dixon E, et al. Impact of regional lymph node evaluation in staging patients with peri-ampullary tumors. Ann Surg Oncol 2007; 14:202–10
Park SJ, Kim SW, Jang JY, et al. Intraoperative transfusion: is it a real prognostic factor of periampullary cancer following pancreatoduodenectomy? World J Surg 2002; 26:487–92
Mariette C, Piessen G, Briez N, et al. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg 2008; 247:365–71
Berger AC, Sigurdson ER, LeVoyer T, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol 2005; 23:8706–12
Nitti D, Marchet A, Olivieri M, et al. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol 2003; 10:1077–85
Le Voyer TE, Sigurdson ER, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol 2003; 21:2912–9
Marchet A, Mocellin S, Ambrosi A, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg 2007; 245:543–52
Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery 2007; 141:610–8
Bassi C, Dervenis C, Butturini G, et al; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005; 138:8–13
Neoptolemos JP, Dunn JA, Stocken DD, et al; European Study Group for Pancreatic Cancer. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet 2001; 358:1576–85
Hamilton SR, Aaltonen LA. WHO Classification of Tumours. Pathology and Genetics of the Digestive System. Lyon: IARC, 2000
Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual, 6th edition. New York: Springer-Verlag, 2002
Sessa F, Furlan D, Zampatti C, et al. Prognostic factors for ampullary adenocarcinomas: tumor stage, tumor histology, tumor location, immunohistochemistry and microsatellite instability. Virchows Arch 2007; 451:649–57
Beghelli S, Orlandini S, Moore PS, et al. Ampulla of vater cancers: T-stage and histological subtype but not Dpc4 expression predict prognosis. Virchows Arch 2002; 441:19–24
Liu N, Liang H, Zhang RP, et al. Number of lymph node metastases: a significant prognostic factor for patients with radical resection of carcinoma of the ampulla of Vater. Zhonghua Wei Chang Wai Ke Za Zhi 2007; 10:350–2
Hsu HP, Shan YS, Hsieh YH, et al. Predictors of recurrence after pancreaticoduodenectomy in ampullary cancer: comparison between non-, early and late recurrence. J Formos Med Assoc 2007; 106:432–43
Lee HY, Choi HJ, Park KJ, et al. Prognostic significance of metastatic lymph node ratio in node-positive colon carcinoma. Ann Surg Oncol 2007; 14:1712–7
Bando E, Yonemura Y, Taniguchi K, et al. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol 2002; 9:775–84
Herr HW. Superiority of ratio based lymph node staging for bladder cancer. J Urol 2003;169:943–5
Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med 2003; 349:2117–27
Wong SL, Ji H, Hollenbeck BK, et al. Hospital lymph node examination rates and survival after resection for colon cancer. JAMA 2007; 298:2149–54
Birkmeyer JD, Sun Y, Wong SL, et al. Hospital volume and late survival after cancer surgery. Ann Surg 2007; 245:777–83
Schrag D, Earle C, Xu F, et al. Associations between hospital and surgeon procedure volumes and patient outcomes after ovarian cancer resection. J Natl Cancer Inst 2006; 98:163–71
Schrag D, Cramer LD, Bach PB, et al. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA 2000; 284:3028–35
Birkmeyer JD, Warshaw AL, Finlayson SR, et al. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery 1999; 126:178–83
Sikora SS, Balachandran P, Dimri K, et al. Adjuvant chemo-radiotherapy in ampullary cancers. Eur J Surg Oncol 2005; 31:158–63
Mehta VK, Fisher GA, Ford JM, et al. Adjuvant chemoradiotherapy for “unfavorable” carcinoma of the ampulla of Vater: preliminary report. Arch Surg 2001; 136:65–9
Acknowledgments
This study was supported by: Fondazione Italiana per le Malattie del Pancreas (FIMP), Fondazione Cariverona, Verona, Italy (Bando 2004); European Community FP6 Program, Grant PL018771 MolDiagPaca; Associazione Italiana Ricerca Cancro (AIRC), Milano, Italy; Fondazione Giorgio Zanotto, Verona, Italy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Falconi, M., Crippa, S., Domínguez, I. et al. Prognostic Relevance of Lymph Node Ratio and Number of Resected Nodes after Curative Resection of Ampulla of Vater Carcinoma. Ann Surg Oncol 15, 3178–3186 (2008). https://doi.org/10.1245/s10434-008-0099-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-008-0099-4