Abstract
Background
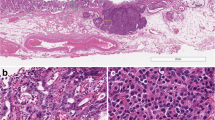
Carcinoid tumors are a group of heterogeneous tumors with neuroendocrine differentiation and are mainly located in the gastrointestinal tract. A high frequency of cytoplasmic accumulation and/or nuclear translocation of β-catenin with frequent mutations of exon 3 of β-catenin gene in gastrointestinal carcinoid tumor has been previously described, but the role of Wnt/β-catenin/APC pathway in the genesis of carcinoid tumor remains largely unknown.
Methods
To further characterize the role of Wnt/β-catenin/APC pathway, we investigated 91 gastrointestinal carcinoid tumors and, for comparison, 26 extragastrointestinal carcinoid tumors by immunohistochemical detection of β-catenin protein and direct sequencing of exon 3 of the β-catenin gene and exon 15 of the APC gene.
Results
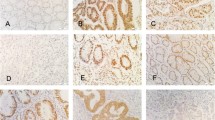
Cytoplasmic accumulation and/or nuclear translocation of β-catenin were found in 27 gastrointestinal carcinoid tumors (29.7%) but not in any extragastrointestinal carcinoid tumors. Interestingly, neither β-catenin nor APC gene mutation was detected in all of the cases with nuclear expression of β-catenin.
Conclusions
Our results indicate that the role β-catenin plays in the genesis of gastrointestinal and extragastrointestinal carcinoid tumors is different. Nuclear expression of β-catenin does not occur in extragastrointestinal carcinoid tumors, and mutation of exon 3 of β-catenin gene and exon 15 of APC gene does not contribute to the activation of Wnt/β-catenin/APC pathway in gastrointestinal carcinoid tumors.

Similar content being viewed by others
References
Bornstein-Quevdo L, Gamboa-Dominguez A. Carcinoid tumour of the duodenum and ampulla of Vater: a clinicomorphologic, immunohistochemical, and cell kinetic comparison. Hum Pathol 2001; 32:1252–6
Hartel M, Wente MN, Sido B, Friess H, Buchler MW. Carcinoid of the ampulla of Vater. J Gastroenterol Hepatol 2005;20:676–81
Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology 2005; 128:1717–51
Soga J. Carcinoids and their variant endocrinomas: an analysis of 11842 reported cases. J Exp Clin Cancer Res 2003; 22:517–30
Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med 1999; 340:858–68
Modlin IM, Shapiro MD, Kidd M. An analysis of rare carcinoid tumors: clarifying these clinical conundrums. World J Surg 2005; 29:92–101
Williams ED, Sandler M. The classification of carcinoid tumors. Lancet 1963; 1:238–9
Solcia E, Kloppel G, Sobin LH. Histological typing of endocrine tumours. In: WHO International Histological Classification of Tumours. Berlin: Springer, 2000; pp 56–74
Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J 1989; 8:1711–7
Bienz M. β-Catenin: a pivot between cell adhesion and Wnt signalling. Curr Biol 2005; 15:R64–7
Rubinfeld B, Souza B, Albert I, et al. Association of the APC gene product with β-catenin. Science 1993; 262:1731–4
Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science 1993; 262:1734–7
Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC–beta-catenin complex and regulation of complex assembly. Science 1996; 272:1023–6
Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation–regulated ubiquitination and degradation of β-catenin. J Biol Chem 1997; 272:24735–8
Behrens J, von Kries JP, Kuhl M, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 1996; 382:638–42
Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R. Nuclear localization of β-catenin by interaction with transcription factor LEF-1. Mech Dev 1996; 59:3–10
Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of β-catenin: evidence for intracellular signaling. J Cell Biol 1995; 128:959–68
Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev 1996; 10:1443–54
Behrens J, Lustig B. The Wnt connection to tumorigenesis. Int J Dev Biol 2004; 48:477–87
Morin PJ, Sparks AB, Korinek V, et al. Activation of β-catenin–Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 1997; 275:1787–90
Moreno-Bueno G, Hardisson D, Sanchez C, et al. Abnormalities of the APC/β-catenin pathway in endometrial cancer. Oncogene 2002; 21:7981–90
Oliva E, Sarrio D, Brachtel EF, et al. High frequency of beta-catenin mutations in borderline endometrioid tumours of the ovary. J Pathol 2006; 208:708–13
Hsu HC, Jeng YM, Mao TL, Chu JS, Lai PL, Peng SY. β-Catenin mutations are associated with a subset of low-stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am J Pathol 2000; 157:763–70
Jeng YM, Wu MZ, Mao TL, Chang MH, Hsu HC. Somatic mutations of β-catenin play a crucial role in the tumorigenesis of sporadic hepatoblastoma. Cancer Lett 2000; 152:45–51
Tejpar S, Nollet F, Li C, et al. Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor). Oncogene 1999; 18:6615–20
Fujimori M, Ikeda S, Shimizu Y, Okajima M, Asahara T. Accumulation of β-catenin protein and mutations in exon 3 of β-catenin gene in gastrointestinal carcinoid tumor. Cancer Res 2001; 61:6656–9
Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci 2006; 119:395–402
Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta–dependent phosphorylation of beta-catenin. EMBO J 1998; 17:1371–84
Ozaki S, Ikeda S, Ishizaki Y, et al. Alterations and correlations of the components in the Wnt signaling pathway and its target genes in breast cancer. Oncol Rep 2005; 14:1437–43
Odajima T, Sasaki Y, Tanaka N, et al. Abnormal beta-catenin expression in oral cancer with no gene mutation: correlation with expression of cyclin D1 and epidermal growth factor receptor, Ki-67 labeling index, and clinicopathological features. Hum Pathol 2005; 36:234–41
Saldanha G, Ghura V, Potter L, Fletcher A. Nuclear beta-catenin in basal cell carcinoma correlates with increased proliferation. Br J Dermatol 2004; 151:157–64
Semba S, Kusumi R, Moriya T, Sasano H. Nuclear accumulation of β-catenin in human endocrine tumors: association with Ki-67 (MIB-1) proliferative activity. Endocrin Pathol 2000; 11:243–50
Mao TL, Chu JS, Jeng YM, Lai PL, Hsu HC. Expression of mutant nuclear β-catenin correlates with non-invasive hepatocellular carcinoma, absence of portal vein spread, and good prognosis. J Pathol 2001; 193:95–101
Huang H, Fujii H, Sankila A, et al. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol 1999; 155:1795–801
Pelosi G, Scarpa A, Puppa G, et al. Alteration of the E-cadherin/beta-catenin cell adhesion system is common in pulmonary neuroendocrine tumors and is an independent predictor of lymph node metastasis in atypical carcinoids. Cancer Lett 2005; 103:1154–64
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Su, MC., Wang, CC., Chen, CC. et al. Nuclear Translocation of β-Catenin Protein but Absence of β-Catenin and APC Mutation in Gastrointestinal Carcinoid Tumor. Ann Surg Oncol 13, 1604–1609 (2006). https://doi.org/10.1245/s10434-006-9072-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-006-9072-2