-
PDF
- Split View
-
Views
-
Cite
Cite
Phioanh (Leia) Nghiemphu, Richard M. Green, Whitney B. Pope, Albert Lai, Timothy F. Cloughesy, Safety of anticoagulation use and bevacizumab in patients with glioma, Neuro-Oncology, Volume 10, Issue 3, June 2008, Pages 355–360, https://doi.org/10.1215/15228517-2008-009
Close - Share Icon Share
Abstract
Bevacizumab in combination with chemotherapy is now being studied for the treatment of malignant gliomas. However, the risk of intracranial hemorrhage has limited its use in patients requiring full anticoagulation for venous thrombosis. To assess the safety of using anticoagulation with bevacizumab, we conducted a retrospective review of our patients who were treated with bevacizumab while receiving anticoagulation. We reviewed their medical records and imaging for signs of hemorrhage. In total, we had 21 patients who received anticoagulation and bevacizumab concurrently for a median time of 72 days. Eighteen patients had adequate anticoagulation for venous thrombosis. There were no frank lobar hemorrhages in any patient. Three patients had small, intraparenchymal hemorrhages on MRI, but only one patient actually developed symptoms due to the hemorrhage. None of these patients had residual neurological deficits from the hemorrhages. Two more patients had evidence of a minor increase in signal on noncontrast T1-weighted sequence, presumed to be petechial hemorrhages, without any clinical sequelae or progression. In contrast, seven patients who had symptomatic hemorrhages from bevacizumab were not on any anticoagulation. In this retrospective review, anticoagulation did not lead to any major hemorrhages and does not appear to be a contraindication for starting bevacizumab therapy.
Bevacizumab is a humanized monoclonal antibody that binds to the vascular endothelial growth factor (VEGF) currently approved for the treatment of metastatic colorectal cancer and non-small-cell lung cancer. This antiangiogenic agent has also been shown to be effective in the treatment of malignant gliomas in combination with chemotherapy.1–3 However, clinical trials with bevacizumab have shown an increased risk of hemorrhage at the site of the primary tumors.4,5 A case study reports intracranial hemorrhages in two patients receiving bevacizumab for systemic cancers while on anticoagulation.6 Intracranial hemorrhages have also been reported in two preliminary reports of using bevacizumab with irinotecan in patients with malignant gliomas, with one hemorrhage leading to death out of 21 patients treated,7 and one grade 2 intracranial hemorrhage in a study of 68 patients.8 At the same time, patients with high-grade gliomas have a higher risk of developing venous thrombosis.9 Clinicians currently have no data to determine whether patients can be on therapeutic anticoagulation and bevacizumab at the same time without an increased risk of hemorrhage. We wanted to assess the safety of anticoagulation with bevacizumab by reviewing the incidence of major intracranial hemorrhage in patients who received anticoagulation at the same time as bevacizumab therapy. To understand whether anticoagulation can exacerbate hemorrhage associated with bevacizumab treatment, we also compared the extent of hemorrhage and clinical deficits to that in patients who developed intracranial hemorrhage while on bevacizumab but not on anticoagulation.
Materials and Methods
We searched the UCLA neurooncology clinical database for patients who had received both bevacizumab and anticoagulation with either warfarin or the low-molecular-weight heparin Lovenox (Sanofi-aventis, Bridgewater, NJ, USA). This database contains clinical data, including treatments, medications, and patient outcome. All patients signed an informed consent form to participate in this database and allow their information to be used in studies, approved by the UCLA and the Kaiser Permanente Los Angeles institutional review boards. We identified 268 patients treated with bevacizumab for glioma at our institutions. Of these patients, 24 also received concurrent anticoagulation. We reviewed their medical records and imaging results to determine their anticoagulation efficacy and for any evidence of intracerebral hemorrhage after starting on both bevacizumab and anticoagulation. Brain imaging was performed using MRI with T1-, T2-, and post contrast T1-weighted images as part of routine follow-up for tumor progression every 4–8 weeks. A few patients also received CT scans of the head. These images were independently reviewed by a neuroradiologist (W.B.P.) for any signs of hemorrhage. We also reviewed the records of seven additional patients who were not on anti coagulation and had known symptomatic hemorrhage while receiving bevacizumab for history of concomitant treatments and clinical outcomes. All hemorrhages were categorized into major hemorrhage with severe neurological deficits, small hemorrhage with some neurological symptoms, and asymptomatic small or petechial hemorrhage.
Results
Patients with Concurrent Anticoagulation
All subjects were treated with bevacizumab at 5 mg/kg every 2 weeks in combination with either a chemotherapy (temozolomide, irinotecan, lomustine, carboplatin, or etoposide) or a molecular treatment (erlotinib or thalidomide). We identified 24 patients at our institutions who had been treated with both bevacizumab and concurrent anticoagulation, but only 21 patients had available imaging for review. Table 1 summarizes their clinical characteristics and types of hemorrhages. Patients were placed on anticoagulation for development of lower-extremity deep venous thrombosis or pulmonary embolism, except for one patient who required anticoagulation for a jugular venous thrombosis and one who required it for an inherited coagulation disorder. Nine of these patients were on the low-molecular-weight heparin Lovenox, while the rest received oral warfarin. Eight of nine patients were given therapeutic dosages of Lovenox (1 mg/kg twice a day). All patients on warfarin achieved an international normalized ratio (INR) of at least 2.0 through most of their treatment period, except for two patients without levels recorded at our institutions. Patients were on both anticoagulation and bevacizumab for a median time of 72 days, with a median of 184 days of follow-up. Further clinical details are given in Table 2 for all patients with hemorrhage.
Summary of clinical characteristics of patients
| Characteristic . | On Anticoagulation (n = 21) . | Hemorrhage, No Anticoagulation (n = 7) . |
|---|---|---|
| Type of hemorrhage | ||
| Major/severe | 0 | 5 |
| Symptomatic small hemorrhage | 1 | 2 |
| Asymptomatic hemorrhage or petechiae | 4 | Unknown |
| Spontaneous T1 changes | 6 | Unknown |
| No hemorrhage | 10 | n/a |
| Pathology | ||
| GBM | 18 | 5 |
| AA | 0 | 0 |
| AMG | 1 | 0 |
| AO | 1 | 1 |
| LA | 1 | 0 |
| LO | 1 | |
| Age–median (range), years | 55 (35–73) | 53 (23–75) |
| Anticoagulation | n/a | |
| LMWH | 9 | |
| Warfarin | 12 | |
| Length of follow-up–median (range), days | 184 (30–501) | 129 (12–301) |
| Characteristic . | On Anticoagulation (n = 21) . | Hemorrhage, No Anticoagulation (n = 7) . |
|---|---|---|
| Type of hemorrhage | ||
| Major/severe | 0 | 5 |
| Symptomatic small hemorrhage | 1 | 2 |
| Asymptomatic hemorrhage or petechiae | 4 | Unknown |
| Spontaneous T1 changes | 6 | Unknown |
| No hemorrhage | 10 | n/a |
| Pathology | ||
| GBM | 18 | 5 |
| AA | 0 | 0 |
| AMG | 1 | 0 |
| AO | 1 | 1 |
| LA | 1 | 0 |
| LO | 1 | |
| Age–median (range), years | 55 (35–73) | 53 (23–75) |
| Anticoagulation | n/a | |
| LMWH | 9 | |
| Warfarin | 12 | |
| Length of follow-up–median (range), days | 184 (30–501) | 129 (12–301) |
Abbreviations: n/a, not applicable; GBM, glioblastoma; AA, anaplastic astrocytoma; AMG, anaplastic mixed glioma; AO, anaplastic oligodendroglioma; LA, low-grade astrocytoma; LO, low-grade oligodendroglioma; LMWH, low-molecular-weight heparin
Summary of clinical characteristics of patients
| Characteristic . | On Anticoagulation (n = 21) . | Hemorrhage, No Anticoagulation (n = 7) . |
|---|---|---|
| Type of hemorrhage | ||
| Major/severe | 0 | 5 |
| Symptomatic small hemorrhage | 1 | 2 |
| Asymptomatic hemorrhage or petechiae | 4 | Unknown |
| Spontaneous T1 changes | 6 | Unknown |
| No hemorrhage | 10 | n/a |
| Pathology | ||
| GBM | 18 | 5 |
| AA | 0 | 0 |
| AMG | 1 | 0 |
| AO | 1 | 1 |
| LA | 1 | 0 |
| LO | 1 | |
| Age–median (range), years | 55 (35–73) | 53 (23–75) |
| Anticoagulation | n/a | |
| LMWH | 9 | |
| Warfarin | 12 | |
| Length of follow-up–median (range), days | 184 (30–501) | 129 (12–301) |
| Characteristic . | On Anticoagulation (n = 21) . | Hemorrhage, No Anticoagulation (n = 7) . |
|---|---|---|
| Type of hemorrhage | ||
| Major/severe | 0 | 5 |
| Symptomatic small hemorrhage | 1 | 2 |
| Asymptomatic hemorrhage or petechiae | 4 | Unknown |
| Spontaneous T1 changes | 6 | Unknown |
| No hemorrhage | 10 | n/a |
| Pathology | ||
| GBM | 18 | 5 |
| AA | 0 | 0 |
| AMG | 1 | 0 |
| AO | 1 | 1 |
| LA | 1 | 0 |
| LO | 1 | |
| Age–median (range), years | 55 (35–73) | 53 (23–75) |
| Anticoagulation | n/a | |
| LMWH | 9 | |
| Warfarin | 12 | |
| Length of follow-up–median (range), days | 184 (30–501) | 129 (12–301) |
Abbreviations: n/a, not applicable; GBM, glioblastoma; AA, anaplastic astrocytoma; AMG, anaplastic mixed glioma; AO, anaplastic oligodendroglioma; LA, low-grade astrocytoma; LO, low-grade oligodendroglioma; LMWH, low-molecular-weight heparin
Clinical characteristics of all patients who developed hemorrhage at the time of the hemorrhage
| Pt # . | Age . | Pathology . | Name of Anticoagulation . | Lovenox Dosage by Weight . | Length on Bevacizumab Prior to Hemorrhage (Days) . | MRI Findings . | Clinical Outcome . | Platelet Count (103/μl) . | INR . | Concurrent Chemotherapy . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients on anticoagulation with hemorrhage | ||||||||||||||||||||
| 1 | 58 | GBM | Lovenox | 2 mg/kg once a day | 39 | Intraparenchymal, peritumoral hemorrhage | Status epilepticus, no deficits | 161 | 1.1 | TAR | ||||||||||
| 2 | 67 | GBM | Lovenox | 1 mg/kg twice a day | 355 | Intraparenchymal, peritumoral hemorrhage | Deficits from tumor progression | 50 | 1.1 | IR | ||||||||||
| 3 | 67 | GBM | Warfarin | 181 | Intraparenchymal, intratumoral hemorrhage | No change | 158 | 7.0 | TMZ | |||||||||||
| 4 | 62 | GBM | Warfarin | 28 | Petechial hemorrhage | No change | 313 | 1.9 | IR | |||||||||||
| 5 | 36 | GBM | Lovenox | 0.9 mg/kg twice a day | 112 | Petechial hemorrhage | No change | 184 | ND | IR | ||||||||||
| Patients with hemorrhage, not on anticoagulation | ||||||||||||||||||||
| 1 | 50 | AO | None | n/a | 35 | Large intraparenchymal hemorrhage | Death | 111 | 1.0 | IR | ||||||||||
| 2 | 52 | GBM | None | n/a | 7 | Large intratumoral hemorrhage | Death | 240 | 1.1 | IR | ||||||||||
| 3 | 62 | GBM | None | n/a | 23 | Peritumoral lobar hemorrhage | Seizure, neuro decline | 173 | ND | IR | ||||||||||
| 4 | 54 | LO | None | n/a | 141 | Intratumoral hemorrhage | Neuro decline | 183 | ND | IR | ||||||||||
| 5 | 56 | GBM | None | n/a | 91 | Intratumoral hemorrhage | Neuro decline | 216 | ND | TMZ | ||||||||||
| 6 | 44 | GBM | None | n/a | 179 | Small intratumoral hemorrhage | Minor neuro decline | ND | ND | IR | ||||||||||
| 7 | 53 | GBM | None | n/a | 149 | Small intratumoral hemorrhage | Minor neuro decline | 229 | ND | CCNU | ||||||||||
| Pt # . | Age . | Pathology . | Name of Anticoagulation . | Lovenox Dosage by Weight . | Length on Bevacizumab Prior to Hemorrhage (Days) . | MRI Findings . | Clinical Outcome . | Platelet Count (103/μl) . | INR . | Concurrent Chemotherapy . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients on anticoagulation with hemorrhage | ||||||||||||||||||||
| 1 | 58 | GBM | Lovenox | 2 mg/kg once a day | 39 | Intraparenchymal, peritumoral hemorrhage | Status epilepticus, no deficits | 161 | 1.1 | TAR | ||||||||||
| 2 | 67 | GBM | Lovenox | 1 mg/kg twice a day | 355 | Intraparenchymal, peritumoral hemorrhage | Deficits from tumor progression | 50 | 1.1 | IR | ||||||||||
| 3 | 67 | GBM | Warfarin | 181 | Intraparenchymal, intratumoral hemorrhage | No change | 158 | 7.0 | TMZ | |||||||||||
| 4 | 62 | GBM | Warfarin | 28 | Petechial hemorrhage | No change | 313 | 1.9 | IR | |||||||||||
| 5 | 36 | GBM | Lovenox | 0.9 mg/kg twice a day | 112 | Petechial hemorrhage | No change | 184 | ND | IR | ||||||||||
| Patients with hemorrhage, not on anticoagulation | ||||||||||||||||||||
| 1 | 50 | AO | None | n/a | 35 | Large intraparenchymal hemorrhage | Death | 111 | 1.0 | IR | ||||||||||
| 2 | 52 | GBM | None | n/a | 7 | Large intratumoral hemorrhage | Death | 240 | 1.1 | IR | ||||||||||
| 3 | 62 | GBM | None | n/a | 23 | Peritumoral lobar hemorrhage | Seizure, neuro decline | 173 | ND | IR | ||||||||||
| 4 | 54 | LO | None | n/a | 141 | Intratumoral hemorrhage | Neuro decline | 183 | ND | IR | ||||||||||
| 5 | 56 | GBM | None | n/a | 91 | Intratumoral hemorrhage | Neuro decline | 216 | ND | TMZ | ||||||||||
| 6 | 44 | GBM | None | n/a | 179 | Small intratumoral hemorrhage | Minor neuro decline | ND | ND | IR | ||||||||||
| 7 | 53 | GBM | None | n/a | 149 | Small intratumoral hemorrhage | Minor neuro decline | 229 | ND | CCNU | ||||||||||
Abbreviations: INR, international normalized ratio; GBM, glioblastoma; TAR, tarceva; IR, irinotecan; TMZ, temozolomide; ND, no data; AO, anaplastic oligodendroglioma; n/a, not applicable; LO, low-grade oligodendroglioma; CCNU, lomustine
Clinical characteristics of all patients who developed hemorrhage at the time of the hemorrhage
| Pt # . | Age . | Pathology . | Name of Anticoagulation . | Lovenox Dosage by Weight . | Length on Bevacizumab Prior to Hemorrhage (Days) . | MRI Findings . | Clinical Outcome . | Platelet Count (103/μl) . | INR . | Concurrent Chemotherapy . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients on anticoagulation with hemorrhage | ||||||||||||||||||||
| 1 | 58 | GBM | Lovenox | 2 mg/kg once a day | 39 | Intraparenchymal, peritumoral hemorrhage | Status epilepticus, no deficits | 161 | 1.1 | TAR | ||||||||||
| 2 | 67 | GBM | Lovenox | 1 mg/kg twice a day | 355 | Intraparenchymal, peritumoral hemorrhage | Deficits from tumor progression | 50 | 1.1 | IR | ||||||||||
| 3 | 67 | GBM | Warfarin | 181 | Intraparenchymal, intratumoral hemorrhage | No change | 158 | 7.0 | TMZ | |||||||||||
| 4 | 62 | GBM | Warfarin | 28 | Petechial hemorrhage | No change | 313 | 1.9 | IR | |||||||||||
| 5 | 36 | GBM | Lovenox | 0.9 mg/kg twice a day | 112 | Petechial hemorrhage | No change | 184 | ND | IR | ||||||||||
| Patients with hemorrhage, not on anticoagulation | ||||||||||||||||||||
| 1 | 50 | AO | None | n/a | 35 | Large intraparenchymal hemorrhage | Death | 111 | 1.0 | IR | ||||||||||
| 2 | 52 | GBM | None | n/a | 7 | Large intratumoral hemorrhage | Death | 240 | 1.1 | IR | ||||||||||
| 3 | 62 | GBM | None | n/a | 23 | Peritumoral lobar hemorrhage | Seizure, neuro decline | 173 | ND | IR | ||||||||||
| 4 | 54 | LO | None | n/a | 141 | Intratumoral hemorrhage | Neuro decline | 183 | ND | IR | ||||||||||
| 5 | 56 | GBM | None | n/a | 91 | Intratumoral hemorrhage | Neuro decline | 216 | ND | TMZ | ||||||||||
| 6 | 44 | GBM | None | n/a | 179 | Small intratumoral hemorrhage | Minor neuro decline | ND | ND | IR | ||||||||||
| 7 | 53 | GBM | None | n/a | 149 | Small intratumoral hemorrhage | Minor neuro decline | 229 | ND | CCNU | ||||||||||
| Pt # . | Age . | Pathology . | Name of Anticoagulation . | Lovenox Dosage by Weight . | Length on Bevacizumab Prior to Hemorrhage (Days) . | MRI Findings . | Clinical Outcome . | Platelet Count (103/μl) . | INR . | Concurrent Chemotherapy . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients on anticoagulation with hemorrhage | ||||||||||||||||||||
| 1 | 58 | GBM | Lovenox | 2 mg/kg once a day | 39 | Intraparenchymal, peritumoral hemorrhage | Status epilepticus, no deficits | 161 | 1.1 | TAR | ||||||||||
| 2 | 67 | GBM | Lovenox | 1 mg/kg twice a day | 355 | Intraparenchymal, peritumoral hemorrhage | Deficits from tumor progression | 50 | 1.1 | IR | ||||||||||
| 3 | 67 | GBM | Warfarin | 181 | Intraparenchymal, intratumoral hemorrhage | No change | 158 | 7.0 | TMZ | |||||||||||
| 4 | 62 | GBM | Warfarin | 28 | Petechial hemorrhage | No change | 313 | 1.9 | IR | |||||||||||
| 5 | 36 | GBM | Lovenox | 0.9 mg/kg twice a day | 112 | Petechial hemorrhage | No change | 184 | ND | IR | ||||||||||
| Patients with hemorrhage, not on anticoagulation | ||||||||||||||||||||
| 1 | 50 | AO | None | n/a | 35 | Large intraparenchymal hemorrhage | Death | 111 | 1.0 | IR | ||||||||||
| 2 | 52 | GBM | None | n/a | 7 | Large intratumoral hemorrhage | Death | 240 | 1.1 | IR | ||||||||||
| 3 | 62 | GBM | None | n/a | 23 | Peritumoral lobar hemorrhage | Seizure, neuro decline | 173 | ND | IR | ||||||||||
| 4 | 54 | LO | None | n/a | 141 | Intratumoral hemorrhage | Neuro decline | 183 | ND | IR | ||||||||||
| 5 | 56 | GBM | None | n/a | 91 | Intratumoral hemorrhage | Neuro decline | 216 | ND | TMZ | ||||||||||
| 6 | 44 | GBM | None | n/a | 179 | Small intratumoral hemorrhage | Minor neuro decline | ND | ND | IR | ||||||||||
| 7 | 53 | GBM | None | n/a | 149 | Small intratumoral hemorrhage | Minor neuro decline | 229 | ND | CCNU | ||||||||||
Abbreviations: INR, international normalized ratio; GBM, glioblastoma; TAR, tarceva; IR, irinotecan; TMZ, temozolomide; ND, no data; AO, anaplastic oligodendroglioma; n/a, not applicable; LO, low-grade oligodendroglioma; CCNU, lomustine
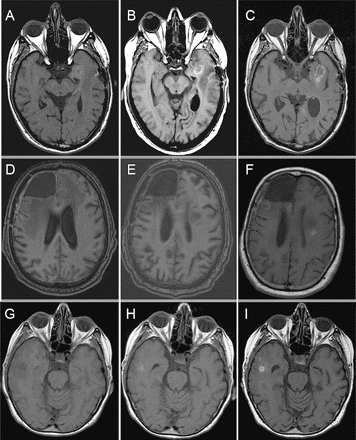
During the treatment period with both bevacizumab and anticoagulation, none of the patients had major lobar hemorrhages that resulted in clinical deficits. Three of 21 patients had definite signs of intraparenchymal hemorrhage on MRI, but only one patient developed symptoms related to the hemorrhage. Patient 1 presented with status epilepticus when a peritumoral hemorrhage developed 39 days after start of treatment. However, the hemorrhage was small and had no increase in mass effect (Fig. 1A–C), and the patient recovered with no neurological sequelae. This patient continued on both bevacizumab and anticoagulation, although the latter at a lower dose,
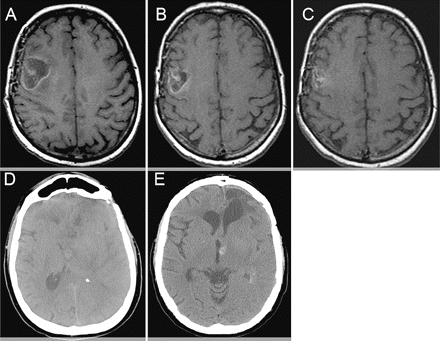
The other two patients had asymptomatic hemorrhages. For patient 2, an intratumoral hemorrhage developed 11 months after start of bevacizumab while the patient was having grade 2 thrombocytopenia. The patient also had tumor progression and had neurological decline more likely related to the progression of his disease, since the hemorrhage did not cause any additional mass effect (Fig. 1D–F). Patient 3 had an asymptomatic, small intraparenchymal hemorrhage at a secondary tumor site about 6 months after treatment without any mass effect (Fig. 1G–I). During the course of treatment, this area was not reported as hemorrhage, and the patient had no clinical symptoms related to this area. This patient remained on both warfarin and bevacizumab without new neurological problems until tumor progression. The hemorrhage was only identified on retrospective review. In addition, two patients also had evidence of noncontrast T1-weighted changes consistent with petechial hemorrhages, one with further progression of hemorrhage along the margin of the resection cavity (patient 5, Fig. 2A–C) and the other with progression into the tumor (patient 6, Fig. 2D, E). Both patients remained asymptomatic.
Other noncontrast T1-weighted imaging changes include spontaneous linear T1 hyperintensity seen on the MRI of six patients that increased mildly over time, but with no obvious evidence of hemorrhage. All these hemorrhages and imaging changes were minor, without associated clinical deficits, mass effect, or edema. All patients continued on treatments with both anticoagulation and bevacizumab despite the imaging changes. Patients were taken off bevacizumab only because of tumor progression and continued with anticoagulation until time of death. Only one patient stopped anticoagulation prior to tumor progression, because of bleeding at the site of an indwelling catheter.
Noncontrast T1-weighted MRI of three patients who developed intraparenchymal hemorrhages while on anticoagulation and bevacizumab. (A–C) Images for patient 1 were obtained prior to treatment (A), 39 days later at the time of symptoms from hemorrhage (B), and 2 months later on follow-up, while still on bevacizumab (C). (D–F) Images of patient 2 were obtained before treatment (D), at 1 month before hemorrhage (E), and at the time of hemorrhage (F). (G–I) Images for patient 3 were obtained prior to (G), 2 months after (H), and 6 months after (I) starting both anticoagulation and bevacizumab.
Patients with Hemorrhage, Not on Anticoagulation
We identified seven patients who had a symptomatic hemorrhage found at the time of their clinical evaluations (Tables 1 and 2). These patients had no history of anticoagulation around the time of their hemorrhage. All patients were treated with bevacizumab at 5 mg/kg, in combination with either chemotherapy or molecular therapy, except for one patient who was on treatment with VEGF-trap, another antiangiogenic therapy, prior to starting bevacizumab (patient 2).
Noncontrast T1-weighted MRI (A–C) and noncontrast head CT (D and E) from two patients with petechial hemorrhages while on anticoagulation and bevacizumab. (A–C) Patient 4 initial scan (A), 1 month after (B), and 4 months after (C) being on both treatments. (D and E) Patient 5 images obtained before (D) and 4 months after (E) both treatments had started.
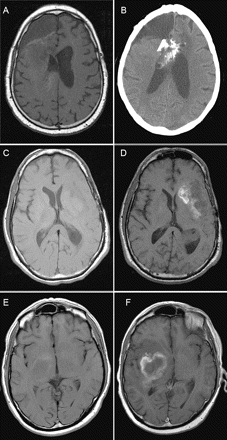
Five patients developed significant hemorrhages, which resulted in clear neurological decline and death in two patients. Patient 1 had a prolonged hospital course from the hemorrhage and died 1 month later from a large lobar hemorrhage (Fig. 3A, B). Patient 2, who was on VEGF-trap in clinical trial, developed a small asymptomatic hemorrhage as well as tumor progression after two treatments and was taken off trial. He then initiated bevacizumab with irinotecan, and the hemorrhage progressed, leading to death (image not available). For patient 3, a lobar hemorrhage (image not available) led to seizures and subsequent neurological decline. Patient 4 had a small but symptomatic hemorrhage after 141 days of bevacizumab (Fig. 3C, D). Treatment was withheld, but the hemorrhage progressed, and the patient had further neurological deficits. Patient 5 had significant neurological decline from both an intratumoral hemorrhage (Fig. 3E, F) and tumor progression. Two other patients (6 and 7) also had mild neurological deficits when they developed small hemorrhages. They continued to receive bevacizumab and had further hemorrhage, and bevacizumab was then withheld. Patients 3–7 died from tumor progression.
Discussion
Overall, we believe that patients can receive anticoagulation at therapeutic dosages while on bevacizumab for treatment. The incidence of hemorrhages seems slightly higher in the group on anticoagulation than in the general population treated with bevacizumab, but our groups are relatively small for statistical determination. Also, since we are only reporting symptomatic hemorrhages in patients not on anticoagulation and do not routinely obtain MR sequences specifically to look for hemorrhage, we may have missed cases of asymptomatic, petechial hemorrhages or spontaneous T1 hyperintense changes in routine clinical practice. In our small group, the addition of anticoagulation to bevacizumab treatment is not associated with major intracerebral hemorrhage with neurological deficits or death. Patients who spontaneously developed severe hemorrhages after bevacizumab were actually not on any anticoagulation. All these patients had either oligodendrogliomas or glioblastomas, the gliomas more frequently associated with hemorrhage. There are no other associations with hemorrhages such as length of treatment or hematological problems. A larger group of patients might identify factors that can increase or decrease the risk of severe hemorrhages from bevacizumab, warranting further studies on hemorrhages associated with bevacizumab.
Representative imaging from patients 1, 4, and 5 with major hemorrhages but not on anticoagulation. (A and B) Patient 1 noncontrast T1-weighted MRIs before bevacizumab (A) and 1 month after (B). (C and D) Noncontrast T1-weighted MRIs for patient 4 obtained before treatment (C) and almost 5 months later (D). (E and F) Images for patient 5 were obtained from before (E) and 3 months after (F) bevacizumab.
Patients with high-grade gliomas often develop petechial hemorrhages and T1-weighted hyperintensities on their MRI from postsurgical changes. From our experience, treatment with anticoagulation, bevacizumab, or both did not necessarily cause severe hemorrhages in these patients. Minor changes on T1-weighted imaging without clinical correlations can also be followed while patients are on these treatments and should not prompt removal of either anticoagulation or bevacizumab. More investigation with head CT or gradient-echo MRI sequences can be considered if there are T1 changes during treatment. At the same time, cautionary measures should be taken in patients on anticoagulation. A lobar hemorrhage that occurs while on anticoagulation can be very difficult to control, and patients on both bevacizumab and anticoagulation need to be followed closely. Patients on warfarin should obtain frequent blood draws for INR level. It may be easier to manage patients on the shorter-acting, low-molecular-weight heparins rather than warfarin, considering that several of our patients reached supertherapeutic levels of warfarin. Overall, patients requiring anticoagulation for venous thrombosis should not be excluded from receiving bevacizumab as a treatment for malignant gliomas.
T.F.C. has served on the Advisory Board of Genentech, Inc.
References
Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy.
Vredenburgh JJ, Desjardins A, Herndon JE II, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma.
Vredenburgh JJ, Desjardins A, Herndon JE II, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme.
Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer.
Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer.
Nguyen T, Abrey L. Intracranial hemorrhage in patients treated with bevacizumab and low-molecular weight heparin.
Stark-Vance V. Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma [abstract]. Proceedings of the World Federation of Neuro-Oncology meeting.
Goli KJ, Desjardins A, Herndon JE, et al. Phase II trial of bevacizumab and irinotecan in the treatment of malignant gliomas. ASCO annual meeting proceedings part I.