-
PDF
- Split View
-
Views
-
Cite
Cite
Matteo Landriscina, Annarita Fabiano, Settimia Altamura, Cinzia Bagalà, Annamaria Piscazzi, Alessandra Cassano, Corrado Spadafora, Francesco Giorgino, Carlo Barone, Mauro Cignarelli, Reverse Transcriptase Inhibitors Down-Regulate Cell Proliferation in Vitro and in Vivo and Restore Thyrotropin Signaling and Iodine Uptake in Human Thyroid Anaplastic Carcinoma, The Journal of Clinical Endocrinology & Metabolism, Volume 90, Issue 10, 1 October 2005, Pages 5663–5671, https://doi.org/10.1210/jc.2005-0367
Close - Share Icon Share
Context: Two classes of repeated genomic elements, retrotransposons and endogenous retroviruses, encode for endogenous nontelomeric reverse transcriptase (RT), a gene that is down-regulated in differentiated cells but is highly expressed in embryonic and transformed tissues. Two nonnucleosidic RT inhibitors, efavirenz and nevirapine, currently used in HIV treatment, reversibly down-regulate tumor growth and induce differentiation in several human tumor cell models.
Objectives: Aggressive biological behavior and loss of specific thyroid cell functions, such as thyroglobulin, thyroid peroxidase, TSH receptor, Na/I symporter expression, and iodine uptake are features of anaplastic thyroid cancer. Thus, we evaluated the use of RT inhibitors as a potentially differentiating and molecular-targeted treatment of this neoplasm.
Results: Our findings showed that nevirapine and efavirenz reversibly inhibit cell proliferation without triggering cell death in the undifferentiated thyroid carcinoma ARO and FRO cells, which exhibited high levels of endogenous RT activity. Inhibition of cell growth was correlated with accumulation of cells in the G0/G1 phase of the cell cycle, with a concomitant decrease in the S phase. Moreover, treated cells demonstrated a differentiated phenotype and a significant reprogramming of gene expression characterized by up-regulation of the TSH receptor, thyroglobulin, thyroid peroxidase, and Na/I symporter genes. Interestingly, RT inhibition reestablished the ability to uptake iodine in response to TSH either in vitro or in vivo and reversibly down-regulated tumor growth in mice xenografts of ARO cells.
Conclusions: These findings support the need for clinical trials to clarify whether RT inhibitors may restore the sensitivity to radiometabolic therapy in anaplastic thyroid tumors.
RETROTRANSPOSABLE ELEMENTS, such as LINE, Alu, and endogenous retroviruses, make up at least 45% of human DNA (1). With the exception of Alu families, all other classes of retroelements are able to retrotranspose autonomously, because all are endowed with a reverse transcriptase (RT)-coding gene (2). Several studies suggest that endogenous RT activity is associated with a variety of either physiological or pathological processes. RT-coding genes are expressed at very low levels in differentiated, nonpathological tissues (3–6), whereas they are up-regulated in embryonic and undifferentiated tissues and in transformed cells (7–11). Together, these findings suggest a direct correlation between the level of expression of RT and the proliferative activity of cells (12).
We have recently observed that nevirapine and efavirenz, two nonnucleosidic RT inhibitors, widely employed in the therapy of AIDS (13), inhibit endogenous RT activity in normal and transformed mammalian cells of different histological origin (14) and induce a specific block in embryo development (15). Furthermore, either the down-regulation of the expression of RT-encoding LINE-1 elements by RNA interference or the pharmacological inhibition of RT activity results in a reversible decrease in the rate of cell growth as well as in the induction of cell differentiation in several human tumor cell lines (14, 16). RT inhibitors induce a significant reprogramming of gene expression that seems to be specific for each cell type and is responsible for the commitment of the cell to differentiate (16). Furthermore, in vivo efavirenz treatment significantly antagonized tumor growth in athymic xenografts of several human tumors (16).
We investigated whether pharmacological modulation of endogenous RT activity may represent a novel approach in the treatment of undifferentiated thyroid cancer, which is often metastatic and is unable to concentrate radioactive iodine (17).
Materials and Methods
Cell cultures
FRO, WRO, and ARO human thyroid carcinoma cells (18) were cultured in DMEM containing 10% fetal bovine serum, glutamine, and penicillin/streptomycin (Sigma-Aldrich, Milan, Italy). Primary cultures of normal thyroid cells were obtained from normal noninfiltrated thyroid gland collected during the surgical removal of a papillary thyroid unifocal carcinoma and processed as previously reported (19). Human recombinant TSH (rTSH) (Sigma-Aldrich) was used at a concentration of 2 mU/ml (20). Nevirapine and efavirenz were purified by Dr. Antonello Mai (University “La Sapienza,” Rome, Italy) from commercially available Viramune (Boehringer-Ingelheim, Ingelheim, Germany) and Sustiva (Bristol-Myers Squibb, New York, NY) and dissolved in dimethylsulfoxide (DMSO). Both drugs, or the same volume of DMSO (0.2%, controls), were added to the cultures. Incubation was carried out continuously; RT inhibitor-containing fresh medium was changed at 48-h intervals. Evaluation of the rate of cell growth, apoptosis, necrosis, and cell cycle analysis was performed as previously reported (16).
Immunoblot analysis and RT activity assay
Cells were lysed in cold lysis buffer [20 mm Tris (pH 7.5) containing 300 mm sucrose, 60 mm KCl, 15 mm NaCl, 5% (vol/vol) glycerol, 2 mm EDTA, 1% (vol/vol) Triton X-100, 1 mm phenylmethylsulfonyl fluoride, 2 mg/ml aprotinin, 2 mg/ml leupeptin and 0.2% (wt/vol) deoxycholate]. Protein concentration was quantified using the Bio-Rad protein assay kit (Bio-Rad Laboratories GmbH, Hercules, CA) according to the manufacturer’s procedures. For thyroglobulin immunoprecipitation, equal amounts of proteins were rotated for 18 h at 4 C in the presence of 1 μg mouse monoclonal antithyroglobulin antibody (Sigma-Aldrich). Protein G-Sepharose (Amersham Pharmacia Biotech, Piscataway, NJ) was added, and the samples were rotated for an additional 2 h at 4 C. Immunoprecipitated proteins were eluted by sample buffer. Equal amounts of proteins from cell lysates or immunoprecipitated proteins were resolved by 6% (wt/vol) SDS-PAGE, transferred to a nitrocellulose membrane (Hybond C; Amersham Pharmacia Biotech), and immunoblotted, respectively, with a mouse monoclonal anti-thyroid peroxidase (TPO) antibody (Alexis Biochemicals, Berne, Switzerland) or a mouse monoclonal antithyroglobulin antibody (Sigma-Aldrich). Specific bands were revealed using the Enhanced Chemiluminescence (ECL) Plus Western Blotting Detection Kit (Amersham Pharmacia Biotech). RT activity was evaluated as previously reported (14).
Indirect immunofluorescence (IF) and confocal laser scanning microscopy.
Treated and untreated cells were fixed with 4% (wt/vol) paraformaldehyde for 30 min and permeabilized in 0.1% (vol/vol) Triton X-100 and 0.1% (vol/vol) Tween 20 in PBS containing 5% (wt/vol) BSA (Sigma-Aldrich) for 1 h. F-actin staining was performed using fluorescein isothiocyanate (FITC)-conjugated phalloidin (Sigma-Aldrich). IF staining was performed using mouse monoclonal antibody against human thyroglobulin (Sigma-Aldrich), TPO (21), and Na/I symporter (NIS) (22) and was revealed by FITC-conjugated IgG secondary antibody (Sigma-Aldrich). Samples were imaged under a confocal Nikon Eclipse TE 2000-S microscope (Nikon, Melville, NY). The excitation and emission wavelengths were 488 and 510 nm, respectively.
RNA extraction and semiquantitative and real-time RT-PCR analysis.
Total RNA was extracted using the Trizol reagent according to the manufacturer’s procedures (Invitrogen, Milan, Italy). For the first-strand synthesis of cDNA, 5 μg RNA were used in a 20-μl reaction mixture using a cDNA Superscript III (Invitrogen) according to the supplier’s instructions. Oligonucleotide primers for semiquantitative and real-time PCR for TSH receptor, thyroglobulin, TPO, NIS, β-actin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are shown in Table 1. Primers for real-time PCR were designed to be intron spanning. For semiquantitative PCR, 1 μl cDNA mixture was withdrawn and amplified using the Taq Gold DNA Polymerase kit (Applied Biosystems, Milan, Italy) in a Gene AMP PCR System 9700 Thermal Cycler (Applied Biosystems). Reaction conditions were 94 C for 10 min, followed by 35 cycles of 30 sec at 94 C, 30 sec at 54 C (NIS at 58 C), 5 min at 72 C, and 7 min at 72 C. β-Actin was chosen as internal control. For quantitative real-time PCR, 1 μl cDNA sample was amplified using the Platinum SYBR Green qPCR Supermix UDG (Invitrogen, Milan, Italy) in an iCycler iQ Real Time Detection System (Bio-Rad Laboratories GmbH). Reaction conditions were 50 C for 2 min, 95 C for 2 min, followed by 50 cycles of 15 sec at 95 C, 30 sec at 56 C (TSHR at 54 C), 30 sec at 72 C. Absolute quantification was carried out against a standard curve performed by amplification of cDNA obtained from normal human thyroid. GAPDH was chosen as internal control.
Sequences of primers
| Genes . | Primers . |
|---|---|
| TSH receptor | |
| Semiquantitative PCR | |
| Sense 1411–1435 | GCCTCTGTAGACCTCTACACTCAC |
| Antisense 2010–2028 | CTTTTCAATCAGTTCATAGACATC |
| Real-time PCR | |
| Sense 93–112 | CCATCAGGAGGAGGACTTCA |
| Antisense 212–231 | ATTGGGCAGATTAGAAAATG |
| Thyroglobulin | |
| Semiquantitative PCR | |
| Sense 2102–2123 | CAGAGTGCTACTGTGTTGATGC |
| Antisense 2787–2808 | TCATCCACACACCAGCAGTTCC |
| TPO | |
| Semiquantitative PCR | |
| Sense 325–344 | ACTCAACAATCACAGCATCC |
| Antisense 725–742 | TGCTCTGTGGTGTGAACG |
| NIS | |
| Semiquantitative PCR | |
| Sense 676–696 | GCCCTCATCCTGAACCAAGTG |
| Antisense 889–907 | TGATCCGGGAGTGGTTCTG |
| Real-time PCR | |
| Sense 1742–1762 | CCATCCTGGATGACAACTTGG |
| Antisense 1821–1841 | AAAAACAGACGATCCTCATTG |
| GAPDH | |
| Real-time PCR | |
| Sense 180–197 | CAAGGCTGAGAACGGGGAA |
| Antisense 250–269 | GCATCGCCCCACTTGATTTT |
| β-Actin | |
| Semiquantitative PCR | |
| Sense 1023–1041 | GGCATCGTGATGGACTCCG |
| Antisense 1824–1842 | GCTGGAAGGTGGACAGCGA |
| Genes . | Primers . |
|---|---|
| TSH receptor | |
| Semiquantitative PCR | |
| Sense 1411–1435 | GCCTCTGTAGACCTCTACACTCAC |
| Antisense 2010–2028 | CTTTTCAATCAGTTCATAGACATC |
| Real-time PCR | |
| Sense 93–112 | CCATCAGGAGGAGGACTTCA |
| Antisense 212–231 | ATTGGGCAGATTAGAAAATG |
| Thyroglobulin | |
| Semiquantitative PCR | |
| Sense 2102–2123 | CAGAGTGCTACTGTGTTGATGC |
| Antisense 2787–2808 | TCATCCACACACCAGCAGTTCC |
| TPO | |
| Semiquantitative PCR | |
| Sense 325–344 | ACTCAACAATCACAGCATCC |
| Antisense 725–742 | TGCTCTGTGGTGTGAACG |
| NIS | |
| Semiquantitative PCR | |
| Sense 676–696 | GCCCTCATCCTGAACCAAGTG |
| Antisense 889–907 | TGATCCGGGAGTGGTTCTG |
| Real-time PCR | |
| Sense 1742–1762 | CCATCCTGGATGACAACTTGG |
| Antisense 1821–1841 | AAAAACAGACGATCCTCATTG |
| GAPDH | |
| Real-time PCR | |
| Sense 180–197 | CAAGGCTGAGAACGGGGAA |
| Antisense 250–269 | GCATCGCCCCACTTGATTTT |
| β-Actin | |
| Semiquantitative PCR | |
| Sense 1023–1041 | GGCATCGTGATGGACTCCG |
| Antisense 1824–1842 | GCTGGAAGGTGGACAGCGA |
Sequences of primers
| Genes . | Primers . |
|---|---|
| TSH receptor | |
| Semiquantitative PCR | |
| Sense 1411–1435 | GCCTCTGTAGACCTCTACACTCAC |
| Antisense 2010–2028 | CTTTTCAATCAGTTCATAGACATC |
| Real-time PCR | |
| Sense 93–112 | CCATCAGGAGGAGGACTTCA |
| Antisense 212–231 | ATTGGGCAGATTAGAAAATG |
| Thyroglobulin | |
| Semiquantitative PCR | |
| Sense 2102–2123 | CAGAGTGCTACTGTGTTGATGC |
| Antisense 2787–2808 | TCATCCACACACCAGCAGTTCC |
| TPO | |
| Semiquantitative PCR | |
| Sense 325–344 | ACTCAACAATCACAGCATCC |
| Antisense 725–742 | TGCTCTGTGGTGTGAACG |
| NIS | |
| Semiquantitative PCR | |
| Sense 676–696 | GCCCTCATCCTGAACCAAGTG |
| Antisense 889–907 | TGATCCGGGAGTGGTTCTG |
| Real-time PCR | |
| Sense 1742–1762 | CCATCCTGGATGACAACTTGG |
| Antisense 1821–1841 | AAAAACAGACGATCCTCATTG |
| GAPDH | |
| Real-time PCR | |
| Sense 180–197 | CAAGGCTGAGAACGGGGAA |
| Antisense 250–269 | GCATCGCCCCACTTGATTTT |
| β-Actin | |
| Semiquantitative PCR | |
| Sense 1023–1041 | GGCATCGTGATGGACTCCG |
| Antisense 1824–1842 | GCTGGAAGGTGGACAGCGA |
| Genes . | Primers . |
|---|---|
| TSH receptor | |
| Semiquantitative PCR | |
| Sense 1411–1435 | GCCTCTGTAGACCTCTACACTCAC |
| Antisense 2010–2028 | CTTTTCAATCAGTTCATAGACATC |
| Real-time PCR | |
| Sense 93–112 | CCATCAGGAGGAGGACTTCA |
| Antisense 212–231 | ATTGGGCAGATTAGAAAATG |
| Thyroglobulin | |
| Semiquantitative PCR | |
| Sense 2102–2123 | CAGAGTGCTACTGTGTTGATGC |
| Antisense 2787–2808 | TCATCCACACACCAGCAGTTCC |
| TPO | |
| Semiquantitative PCR | |
| Sense 325–344 | ACTCAACAATCACAGCATCC |
| Antisense 725–742 | TGCTCTGTGGTGTGAACG |
| NIS | |
| Semiquantitative PCR | |
| Sense 676–696 | GCCCTCATCCTGAACCAAGTG |
| Antisense 889–907 | TGATCCGGGAGTGGTTCTG |
| Real-time PCR | |
| Sense 1742–1762 | CCATCCTGGATGACAACTTGG |
| Antisense 1821–1841 | AAAAACAGACGATCCTCATTG |
| GAPDH | |
| Real-time PCR | |
| Sense 180–197 | CAAGGCTGAGAACGGGGAA |
| Antisense 250–269 | GCATCGCCCCACTTGATTTT |
| β-Actin | |
| Semiquantitative PCR | |
| Sense 1023–1041 | GGCATCGTGATGGACTCCG |
| Antisense 1824–1842 | GCTGGAAGGTGGACAGCGA |
In vitro iodine uptake assay.
Iodine uptake assay was performed as reported by Schmutzler et al. (20). ARO and FRO cells were incubated for 10 d in the presence and absence of, respectively, 10 and 20 μm efavirenz and 350 μm nevirapine, then harvested, counted, plated in 24-well plates, and further incubated for 48 h in the same conditions in the presence and absence of 2 mU/ml human rTSH. For the assay, the medium was removed and washed with 1 ml HBSS (137 mm NaCl, 5.4 mm KCl, 1.3 mm CaCl2, 0.4 mm MgSO4, 0.5 mm MgCl2, 0.4 mm Na2HPO4, 0.44 mm KH2PO4, 5.55 mm glucose, 10 mm HEPES, pH 7.3). Cells were overlaid with HBSS containing 10 μm NaI and carrier-free Na125I to give a specific activity of 20 mCi/mmol. To control the specific uptake, some of the reactions were supplemented with the NIS inhibitor NaClO4 (10 μm). After 30 min at 37 C in a humid atmosphere, cells were washed with ice-cold HBSS, and accumulated iodine was extracted at –20 C with 1 ml ethanol. Ethanol extracts were counted in a Packard Cobra II Auto-γ counter (PerkinElmer, Wellesley, MA). In parallel cell cultures, incubated in the same conditions, cells were harvested and counted in a Burker chamber (Sigma-Aldrich). Iodine uptake was normalized by cell numbers and expressed as cpm/100,000 cells. Results represent the average (±sd) of three experiments, each in quadruplicate.
Tumor xenografts, animal treatment, and in vivo iodine uptake assay.
Athymic nude 4-wk-old mice (Harlan, Milan, Italy) were kept in accordance with European community guidelines. Mice were inoculated sc in the lower back with ARO cells (1 × 106/mouse) suspended in PBS and treated as previously reported (16). The analysis of iodine accumulation in vivo was performed as previously reported (23). Three weeks after tumor implant, animals were injected im, twice every 24 h, with 0.5 μg of human rTSH. On the day after the last injection, 10 μCi Na125I were delivered ip. Animals were killed 4, 24, and 48 h after Na125I injection; their tumor and organs were removed and weighed. Iodine uptake was measured in a Packard Cobra II Auto-γ counter (PerkinElmer), normalized by weight, and expressed as a ratio between tumor and thyroid radioactivity.
Results
Human undifferentiated thyroid tumor cells exhibit high levels of RT activity
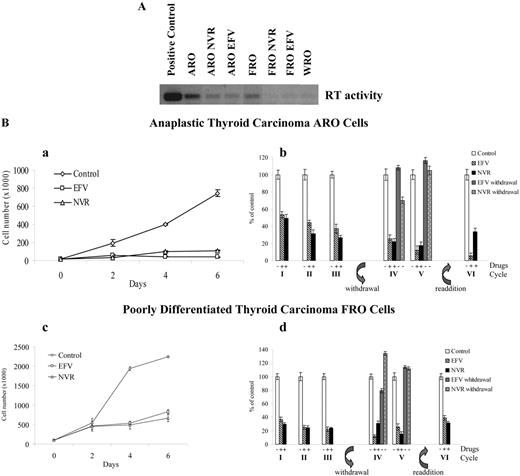
The endogenous RT enzymatic activity was functionally tested in an RT-PCR in vitro assay (14) using cell lysates as a source of endogenous RT and purified MS2 phage RNA as template. Cell lysates obtained from either anaplastic ARO or poorly differentiated FRO thyroid tumor cells were able to reverse transcribe the MS2 phage RNA, obtaining a DNA band of the expected molecular weight (Fig. 1A). Interestingly, anaplastic thyroid tumor ARO cells exhibited higher levels of endogenous RT activity than undifferentiated thyroid tumor FRO cells, whereas differentiated thyroid tumor WRO cells did not reveal detectable levels of RT activity. Moreover, the treatment of ARO and FRO cells with 350 μm nevirapine and 20 μm efavirenz significantly down-regulated the levels of endogenous RT (Fig. 1A). These findings suggest that undifferentiated thyroid tumor cells are characterized by high levels of RT activity and that this activity is down-regulated in the presence of pharmacological inhibitors of RT.
A, Undifferentiated thyroid tumor ARO and FRO cells exhibit high levels of endogenous RT activity. ARO, FRO, and WRO cells were incubated in the presence of DMSO, 350 μm nevirapine (ARO NVR and FRO NVR), or 20 μm efavirenz (ARO EFV and FRO EFV). Endogenous RT activity was evaluated as reported in Ref.14 . In the positive control reaction, the cell extract was substituted with commercial RT. B, RT inhibitors reversibly down-regulate cell proliferation in undifferentiated thyroid tumor ARO and FRO cells. ARO (a) and FRO (c) cells were plated in six-well plates, incubated in the presence and absence of, respectively, 10 and 20 μm EFV or in the presence of 350 μm NVR, harvested every 2 d, and counted in a Burker chamber. Data are reported as absolute cell numbers. ARO (b) and FRO (d) cells were incubated in the presence and absence of, respectively, 10 and 20 μm EFV or in the presence of 350 μm NVR and were cultured through three subsequent cycles of 96 h each. At the end of each cycle, cells were harvested, counted in a Burker chamber, and replated at equal densities. Afterward, pretreated cells were incubated in the presence of the same concentrations of efavirenz and nevirapine or in a drug-free medium for two additional 96-h cycles. Finally, cells were resupplemented with either drug for an additional cycle. Data are reported as percentage of control cells.
RT inhibitors reversibly down-regulate the rate of cell growth in human undifferentiated thyroid tumor cells without inducing apoptosis or necrosis
To reveal the rate of apoptosis and necrosis in ARO and FRO thyroid carcinoma exposed to RT inhibitors, cells were incubated in the presence and absence of 5, 10, 20, and 40 μm efavirenz or 200, 350, and 500 μm nevirapine, harvested after 72 h and stained with Hoechst 33258 and propidium iodate. We did not observe any increase in cell death in the presence of 1) 10 μm efavirenz in ARO cells, 2) 20 μm efavirenz in FRO cells, and 3) 350 μm nevirapine in both cell lines. By contrast, a 15–20% and 5–10% induction, respectively, of apoptosis and necrosis were observed with higher doses of either drug. Of note, these noncytotoxic concentrations of efavirenz (10–20 μm) and nevirapine (350 μm) are in the therapeutic range of both drugs (24). Thus, ARO and FRO thyroid tumor cells were cultured in the presence and absence of, respectively, 10 or 20 μm efavirenz or 350 μm nevirapine, harvested every 2 d, and counted. As reported in Fig. 1B, the growth rate of ARO (a) and FRO (c) cells was significantly inhibited by either drug.
Furthermore, we addressed the question of whether RT-dependent inhibition of cell growth is reversible. Both cell lines were incubated in the presence and absence of either drug and cultured for three 96-h cycles. Cells were harvested every cycle, counted, and replated at equal densities. The continuous incubation of cells with either drug produced about 60–70% inhibition of the rate of cell proliferation (Fig. 1B, b and d). Afterward, pretreated cells were incubated in the presence of either efavirenz or nevirapine or else in a drug-free medium. The withdrawal of RT inhibitors resulted in a prompt recovery of cell proliferation with a rate of cell growth similar to control cells. Moreover, the down-regulation of cell proliferation was reinduced when the same ARO and FRO cell cultures were resupplemented with RT inhibitors (Fig. 1B, b and d). These findings suggest that RT may be involved in the regulation of cell proliferation in undifferentiated thyroid cancer cells.
RT inhibitors enhance the percentage of ARO and FRO cells in the G0-G1 phase of cell cycle
We further investigated the cell cycle distribution in thyroid tumor cell lines treated with efavirenz and nevirapine. Both RT inhibitors induced a significant increase in the percentage of cells in the G0-G1 phase of the cell cycle from 39.3 ± 0.9 in control ARO cells to 62.4 ± 2.8 and 65.9 ± 2.4 in ARO cells exposed, respectively, to nevirapine and efavirenz and from 32.9 ± 2.7 in control FRO cells to 46.9 ± 3.0 and 42.8 ± 3.4, respectively, in nevirapine- and efavirenz-treated FRO cells. Moreover, we observed a parallel decrease in cells in the S phase from 50.2 ± 2.2 in control ARO cells to 23.2 ± 2.9 and 25.8 ± 1.0 in ARO cells treated, respectively, with nevirapine and efavirenz and from 39.5 ± 1.2 in control FRO cells to 24.1 ± 2.3 and 22.6 ± 1.9 in FRO cells exposed, respectively, to nevirapine and efavirenz. Interestingly, the withdrawal of efavirenz and nevirapine from the cell culture for 4 d resulted in the loss of the G0-G1 phase fraction accumulation. This observation suggests that down-regulation of RT activity correlates with the arrest of cells in the G0 phase or with a delay in G1-S transition.
RT inhibitors induce cell differentiation in human anaplastic thyroid tumor cells
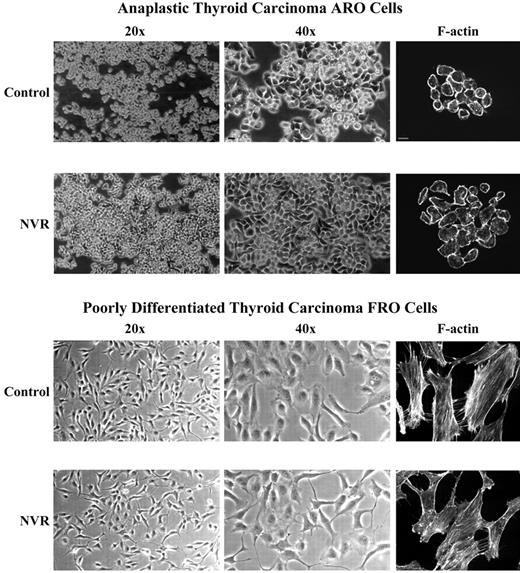
Thyroid tumor ARO cells exhibit several morphological features representative of anaplastic tumors, such as high proliferation rate and formation of cell clusters organized into multilayer populations, whereas the poorly differentiated thyroid carcinoma FRO cells are devoid of most of these features (Fig. 2). We questioned whether RT inhibition is able to revert the morphological features of anaplasia in ARO cells, as previously demonstrated in human melanoma and prostate carcinoma cells treated with RT inhibitors (16). Thus, ARO and FRO cells were incubated in the presence of DMSO or 350 μm nevirapine for 4 d, harvested, replated at high density, and further cultured for 2 d in the same conditions. Nevirapine treatment induced in anaplastic thyroid tumor ARO cells 1) a more flattened phenotype, 2) an increase in cell adhesion, and 3) restoration of monolayer cell growth with a significant reduction in cluster formation (Fig. 2). By contrast, nevirapine induced only minimal morphological changes in the poorly differentiated thyroid tumor FRO cell line, with the appearance of elongated dendritic extensions tightly adherent to the substrate (Fig. 2). Similar results were obtained when the same cell lines were treated with efavirenz (data not shown). These morphological changes suggest that RT inhibitors may induce cell differentiation in anaplastic thyroid tumor cells.
Nevirapine (NVR) induces morphological differentiation in anaplastic thyroid tumor ARO cells. ARO and FRO cells were cultured in the presence of DMSO (Control) or 350 μm NVR for 4 d, harvested, counted, replated at high density in six-well plates, and further incubated in the same medium for 2 d. Photographs were obtained by phase contrast microscopy using ×20 and ×40 objectives. F-actin staining was performed using FITC-conjugated phalloidin and imaged under a confocal Nikon microscope. Bars, 20 μm.
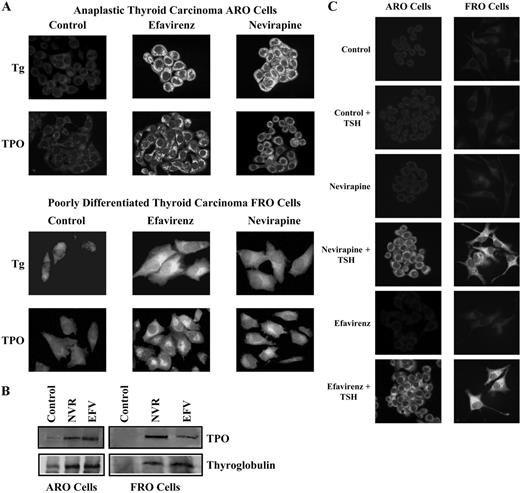
We further investigated the RT-dependent differentiation of thyroid tumor cell lines by analyzing specific products of differentiated thyrocytes. Thus, ARO and FRO cells were exposed to efavirenz and nevirapine for 10 d, stained with antithyroglobulin and anti-TPO antibodies, and analyzed by indirect IF. As reported in Fig. 3A, IF analysis demonstrated undetectable expression of thyroglobulin in both ARO and FRO control cells. By contrast, exposure to efavirenz or nevirapine resulted in a significant increase in thyroglobulin levels. Consistently, ARO and FRO untreated cells exhibited undetectable levels of TPO, whereas the exposure of ARO and FRO cells to efavirenz or nevirapine induced a significant increase in the expression of TPO. Furthermore, TPO immunoblot analysis exhibited the expected 110-kDa band in ARO and FRO cells treated with either efavirenz or nevirapine for 10 d, but not in DMSO-treated controls (Fig. 3B). Similarly, thyroglobulin immunoprecipitation from ARO and FRO cells revealed a strong up-regulation of the expected 330-kDa band in cells exposed to efavirenz and nevirapine for 10 d (Fig. 3B). These results suggest that RT inhibitors can facilitate the onset of cell differentiation in undifferentiated thyroid tumor cell lines, as thyroglobulin and TPO genes are highly expressed by normal thyroid cells and thyroid differentiated tumors but not by anaplastic thyroid carcinoma cells (25).
RT inhibitors induce the expression of thyroglobulin, TPO, and NIS in undifferentiated thyroid tumor ARO and FRO cells. A, ARO and FRO cells were incubated in the presence of DMSO (Control), efavirenz, or nevirapine for 10 d, fixed, permeabilized, and stained by a mouse monoclonal antibody against human thyroglobulin (Tg) or human TPO. Specific signal was revealed by FITC-conjugated IgG secondary antibody and imaged under a confocal Nikon microscope. B, ARO and FRO cells were incubated in the presence of DMSO (Control), efavirenz (EFV), or nevirapine (NVR) for 10 d. Total cell lysates or immunoprecipitated proteins were separated by 6% SDS-PAGE and immunoblotted, respectively, by mouse monoclonal anti-TPO or antithyroglobulin antibodies. C, ARO and FRO cells were incubated in the presence of DMSO (Control), efavirenz, or nevirapine for 4 d, stimulated with 2 mU/ml human rTSH in the presence of either drug, fixed, permeabilized, and stained by a mouse monoclonal antibody against human NIS. Specific signal was revealed by FITC-conjugated IgG secondary antibody and imaged under a confocal Nikon microscope.
RT inhibitors induce the expression of TSH receptors and restore the ability of TSH to up-regulate NIS expression in anaplastic thyroid tumors
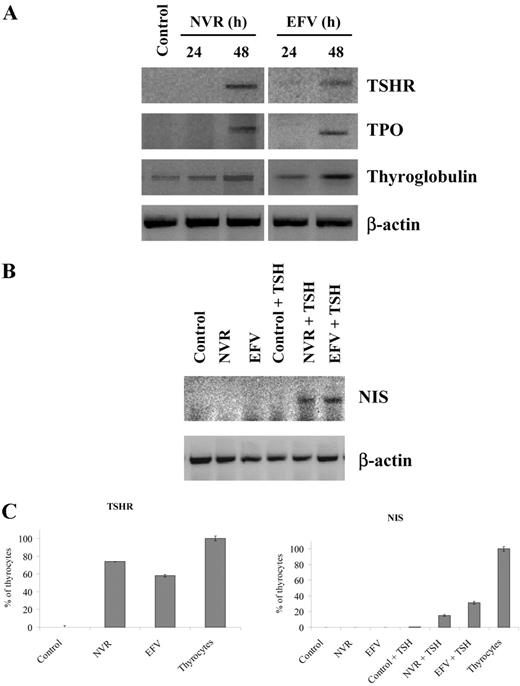
It is well known that anaplastic thyroid tumors are characterized by reduced or absent expression of TSH receptors as well as reduced signal transduction after receptor activation (17). Thus, we questioned whether the cell differentiation obtained by pharmacological inhibition of RT was correlated with the up-regulation of TSH receptor gene expression. This was investigated by semiquantitative RT-PCR in cultures of anaplastic thyroid carcinoma ARO cells exposed to either 350 μm nevirapine or 10 μm efavirenz for 24 and 48 h. We observed that 48-h exposure to nevirapine or efavirenz induced a significant up-regulation of TSH receptor gene expression (Fig. 4A). In parallel, we also evaluated the expression of thyroglobulin and TPO genes and observed that the expression of either gene is strongly induced in nevirapine- and efavirenz-treated ARO cells. Interestingly, the up-regulation of either gene occurred within 48 h of exposure to nevirapine or efavirenz, as observed for the TSH receptor gene (Fig. 4A).
RT inhibitors up-regulate TSH receptor and NIS gene expression and restore TSH signaling in anaplastic thyroid carcinoma ARO cells. A, Total RNA was extracted from ARO cells incubated for 24 and 48 h in the presence and absence of 350 μm nevirapine (NVR) or 10 μm efavirenz (EFV) and amplified by semiquantitative RT-PCR, using primers specific for TSH receptor (TSHR), thyroglobulin, and TPO genes. β-Actin was used as internal standard. B, ARO cells were incubated for 96 h in the presence and absence of 350 μm NVR or 10 μm EFV and then stimulated with 2 mU/ml human rTSH in the presence of either drug. Total RNA was amplified by semiquantitative RT-PCR using primers specific for the NIS gene. C, Total RNA was extracted either from ARO cells, exposed to 350 mm NVR or 10 μm EFV in the presence and absence of rTSH, or from normal thyrocytes and amplified by real time RT-PCR, using primers specific for the TSHR and NIS genes. GAPDH was used as internal standard. Gene expression was expressed as a percentage of that observed in normal thyrocytes.
It is also well established that normal thyroid cells and differentiated thyroid tumors are able to accumulate iodine due to the expression of NIS, a gene under the control of TSH receptor signaling (25), whereas poorly differentiated and anaplastic thyroid tumors are devoid of NIS expression (17, 25). Because RT inhibitors induce differentiation in anaplastic thyroid tumor cell lines by restoring expression of the TSH receptor, we further questioned whether efavirenz and nevirapine are able to up-regulate NIS expression in a TSH-dependent manner. Therefore, ARO cells were exposed to either RT inhibitor for 4 d and then were further stimulated by TSH in the presence and absence of either drug for another 2 d. As reported in Fig. 4B, ARO cells expressed negligible levels of NIS mRNA and were not able to up-regulate the NIS gene in response to TSH. Interestingly, the basal expression of NIS was not influenced by either RT inhibitor, whereas it was significantly induced by TSH in nevirapine- and efavirenz-pretreated cells. Of note, a similar RT-dependent induction of TSH receptor, NIS, thyroglobulin, and TPO genes was also observed in FRO cells (data not shown). Consistently, IF analysis with anti-NIS antibodies in ARO and FRO cells revealed that only cells exposed to nevirapine or efavirenz, and not control cells, were able to up-regulate NIS protein levels in response to TSH (Fig. 3C). These data suggest that RT inhibitors are able to induce a specific reprogramming of gene expression in anaplastic thyroid carcinoma cells, resulting in the reestablishment of TSH signaling.
To compare the levels of expression of these thyroid-specific genes in redifferentiated ARO tumor cells with the constitutive levels of expression of the same genes in normal thyroid cells, real-time PCR was used to evaluate the expression of the TSH receptor gene in cells exposed to efavirenz, nevirapine, or DMSO for 48 h and the expression of the NIS gene in cells treated with nevirapine or efavirenz for 4 d and further stimulated with TSH for 2 d. Primary cultures of human thyrocytes were used as controls. We observed that, in comparison to control ARO cells, nevirapine and efavirenz up-regulated the TSH receptor, respectively, 7.4 and 5.8 times. Similarly, TSH was able to induce NIS expression in nevirapine- and efavirenz-pretreated cells by, respectively, 58.5 and 121.1 times in comparison with ARO control cells stimulated with TSH. Furthermore, the nevirapine- and efavirenz-induced up-regulation of the TSH receptor was, respectively, 74.1 and 58.1% of that observed in human thyrocytes, whereas the induction of NIS expression was, respectively, 15.2 and 31.5% of that observed in normal thyroid cells.
RT inhibitors restore the ability to accumulate radioactive iodine in human undifferentiated thyroid tumors
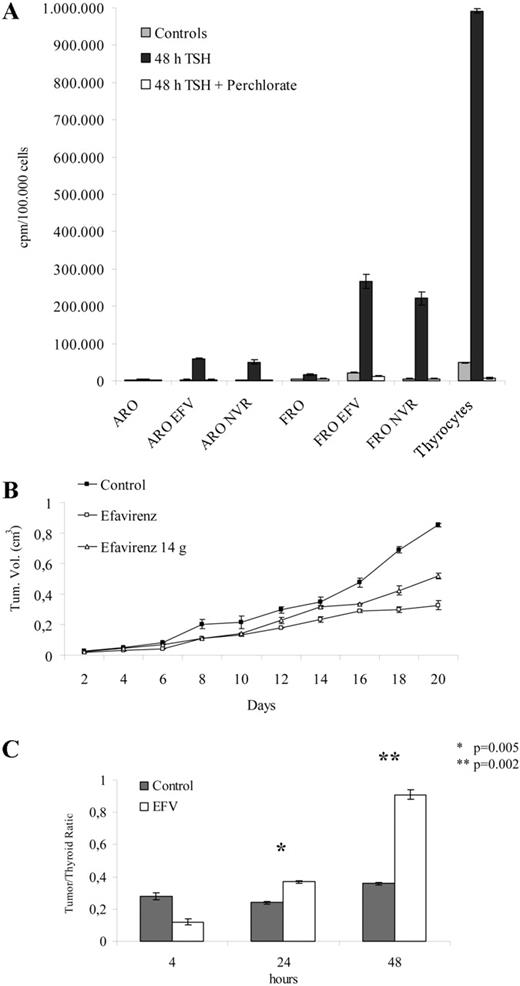
Because NIS is responsible for iodine uptake in response to TSH stimulation in thyroid cells (25), we evaluated whether ARO and FRO cells treated with RT inhibitors acquire the ability to accumulate radioactive iodine. Cells were exposed to RT inhibitors for 10 d, stimulated for 48 h with TSH to obtain the induction of the NIS gene, and further incubated in the presence of radioactive iodine. Primary cultures of normal thyroid cells were used as controls. ARO and FRO control cells exhibited minimal ability to accumulate iodine in response to TSH stimulation. Interestingly, the pretreatment of cells with efavirenz and nevirapine elicited a strong increase in iodine uptake in response to TSH (Fig. 5A). If iodine uptake was compared with the respective unstimulated controls, efavirenz treatment increased iodine uptake by about 16–17 times in ARO cells and 10 times in FRO cells, whereas nevirapine elicited a more marked increase—about 26–27 times in ARO cells and 40 times in FRO cells. The efavirenz- and nevirapine-dependent up-regulation of TSH-stimulated iodine uptake was sensitive to the NIS inhibitor, sodium perchlorate (Fig. 5A). Of note, TSH stimulation strongly up-regulated iodine uptake in normal thyroid cells, and this induction was about 3.8 times higher than that elicited by TSH in nevirapine-treated FRO cells (Fig. 5A). This ability of RT inhibitors to restore iodine uptake in anaplastic thyroid tumor cells suggests that the RT-dependent reprogramming of gene expression is able to regulate functions that are typical of differentiated cells.
RT inhibitors restore iodine uptake in undifferentiated thyroid tumor cells either in vitro or in vivo and down-regulate the growth of anaplastic thyroid tumor xenografts in athymic mice. A, ARO and FRO cells were incubated in the presence and absence of, respectively, 10 and 20 μm EFV or in the presence of 350 μm NVR for 10 d and were then harvested, counted, plated in 24-well plates, and further incubated in the same conditions in the presence and absence of 2 mU/ml human rTSH for 48 h. Primary cultures of human thyrocytes were used as positive controls and incubated in the presence and absence of 2 mU/ml human rTSH for 48 h. For the assay, the medium was removed and incubated in HBSS containing 10 μm NaI and carrier-free Na125I. Some of the reactions received this assay buffer, supplemented with the NIS inhibitor NaClO4, to control the specific uptake. Accumulated iodine was extracted with ethanol at −20 C and counted in a gamma counter. Results are normalized by cell numbers and are expressed as counts per minute per 100,000 cells. B, ARO cells were inoculated in athymic mice and injected 5 d/wk with 20 mg/kg EFV starting 1 d after tumor xenografts (open squares) or with DMSO (closed squares). In a group of animals, the treatment was discontinued 14 d after tumor injection (open triangles). Tumor growth was monitored by caliper measurements and reported as tumor volumes. C, Mice xenografts of ARO cells were continuously treated with 20 mg/kg EFV for 3 wk, stimulated twice with 0.5 μg of human rTSH every 24 h; on the day after the last injection, 10 μCi Na125I was delivered ip. Animals were killed 4, 24, and 48 h after radioactive iodine injection; iodine uptake was measured in tumors and organs, normalized by weight, and expressed as a ratio between tumor and thyroid radioactivity.
RT inhibitors down-regulate tumor growth and induce iodine accumulation in mouse xenografts of ARO cell lines
Because ARO cells are well known to be highly tumorigenic in host animals (26), we evaluated the ability of efavirenz to inhibit thyroid tumor growth and induce iodine uptake in vivo. After inoculation of ARO cells in athymic mice, the animals were subjected to anti-RT treatment with efavirenz, and tumor size was determined every other day. Animals were treated by sc injection of efavirenz (20 mg per kilogram of body weight; 5 d/wk), starting one day after tumor inoculation (16). A 50% reduction in the growth rate was recorded in efavirenz-treated mice compared with untreated tumors (Fig. 5B). We also evaluated ARO cell-derived tumor growth in animals treated with efavirenz starting 1 d after the inoculation, but discontinuing the treatment after 14 d. In agreement with the results obtained in vitro, these experiments showed that inhibition of RT-dependent tumor growth is reversible in vivo, as it is resumed in inoculated animals in which the drug treatment was interrupted (Fig. 5B). These data indicate that RT inhibition reversibly antagonizes the growth of anaplastic thyroid tumors in vivo.
To evaluate iodine accumulation in ARO cells implanted in athymic mice, animals were continuously treated with DMSO or efavirenz for 3 wk, stimulated with human rTSH, and then injected with Na125I. Iodine uptake in tumors was analyzed 4, 24, and 48 h later and was expressed as the ratio between tumor and thyroid radioactivity. As reported in Fig. 5C, efavirenz-treated tumors exhibited a progressive increase in the ratio of iodine accumulation between tumor and thyroid that was maximal 48 h after radioactive iodine injection, while control tumors exhibited a constant low level of iodine accumulation. This ability of RT inhibitors to restore iodine uptake in anaplastic thyroid tumors both in vitro and in vivo suggests that these drugs may tentatively be used to enhance the sensitivity to radiometabolic therapy in humans.
Discussion
In thyroid epithelial carcinomas, differentiation refers to the maintenance of cellular functions that are characteristic of normal thyroid follicular cells. Moreover, the degree of differentiation among thyroid tumors determines the likelihood of a beneficial response to therapeutic options that take advantage of thyroid-specific processes, such as iodine uptake and organification. Differentiated thyroid tumor cells express cell membrane receptors for TSH with active transduction machinery that stimulates progression through the cell cycle, elaboration of thyroglobulin, and both production and membrane-targeting of NIS. This provides the rationale for transient stimulation of thyroid tumors with rTSH to induce 131I delivery to malignant cells for both diagnostic and therapeutic purposes (17). The ability to treat metastatic disease with radioactive iodine is unique to thyroid cancer with sufficient expression of NIS (27). Poorly differentiated and anaplastic thyroid tumors are devoid of these cellular functions and are characterized by an aggressive biological behavior, short clinical doubling time, and invariably metastatic dissemination. The loss of thyroid-specific functions impedes both diagnostic and therapeutic efforts, due to the tumors’ inability to express NIS and concentrate radioactive iodine. Dedifferentiation may result in the diminished expression of TSH receptors as well as diminished signal transduction after receptor activation. It appears likely that some of these changes that characterize the dedifferentiation of thyroid tumors, particularly epigenetic changes, may be reversible (17, 28).
The data reported in this study demonstrate that, in undifferentiated thyroid tumors, RT inhibitors produce 1) a reversible down-regulation of cell proliferation in vitro, 2) a reversible inhibition of tumor growth in mice xenografts, 3) an induction of cell differentiation, and 4) a reestablishment of TSH signaling and iodine uptake in vitro and in vivo. It is noteworthy that pharmacological agents are able to induce either cell differentiation and NIS expression or radioactive iodine uptake in undifferentiated thyroid tumors. Interestingly, besides normal thyroid cells and differentiated thyroid tumors, several other tissues express NIS at low levels, but this appears to be insufficient to retain radioiodine within the tumor cells long enough to deliver tumoricidal radiation doses, as shown by NIS transfection studies (29). Retention requires the organification of iodine, mediated by TPO (17). Thyroid TPO expression is diminished by malignant transformation (30) and may account for the rapid loss of accumulated radioiodine, which is probably responsible for some treatment failures (31). Retinoic acid (RA) was one of the earliest compounds tested in undifferentiated thyroid tumors with the aim of facilitating iodine uptake. Indeed, RA elicited a strong up-regulation of NIS expression in ARO cells but failed to induce iodine uptake in vitro and in vivo (20, 32). Moreover, anaplastic thyroid tumor cell lines are devoid of RA receptor β, which is likely responsible for the differentiating and antiproliferative activity of RA in differentiated thyroid tumor cells (33). Recently, histone deacetylase inhibitors have been demonstrated to induce the expression of thyroid specific genes and induce radioiodine accumulation in anaplastic thyroid tumor cells (23).
The ability of RT inhibitors to reestablish functional TSH signaling by simultaneously inducing the expression of TSH receptor, thyroglobulin, and TPO genes and the ability to respond to TSH stimulation with the up-regulation of NIS expression and iodine uptake is, to our knowledge, the first evidence that the pharmacological inhibition of RT activity is able to induce a substantial reprogramming of cell fate in undifferentiated human tumor cells and restore functions that are typical of differentiated cells. Thus, these findings support the hypothesis that endogenous RT may represent a functional “marker” of the cellular machinery associated with high proliferation and loss of differentiation. Finally, inhibition of RT in undifferentiated thyroid tumors may be a novel molecular-targeted differentiating treatment that may be tentatively used to restore sensitivity to radiometabolic therapy. Thus, specifically designed clinical trials are needed to evaluate this hypothesis.
The authors thank Anthony Green for kindly revising the manuscript and Claudia De Tullio for expert technical support.
This work was supported by PRIN Grant no. 2002063999_005 (to M.C.) and by Progetti di Rilevanza Nazionale Grant no. 2004054004_002 (to M.L).
First Published Online July 19, 2005
Abbreviations:
- DMSO,
Dimethylsulfoxide;
- FITC,
fluorescein isothiocyanate;
- GAPDH,
glyceraldehyde-3-phosphate dehydrogenase;
- IF,
immunofluorescence;
- NIS,
Na/I symporter;
- RA,
retinoic acid;
- RT,
reverse transcriptase;
- rTSH,
recombinant TSH;
- TPO,
thyroid peroxidase.