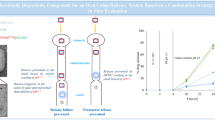
Abstract
Over the past decades, increasing interests took place in the realm of drug delivery systems. Beyond treating intestinal diseases such as inflammatory bowel disease, colon targeting can provide possible applications for oral administration of proteins as well as vaccines due to the lower enzymatic activity in the distal part of GIT. To date, many strategies are employed to reach the colon. This article encompasses different biomaterials tested as film coatings and highlights appropriate formulations for colonic drug delivery. A comparison of different films was made to display the most interesting drug release profiles. These films contained ethylcellulose, as a thermoplastic polymer, blended with an aqueous shellac ammonium salt solution. Different blend ratios were selected as well for thin films as for coated mini-tablets, mainly varying as follows: (80:20); (75:25); (60:40). The impact of blend ratio and coating level was examined as well as the addition of natural polysaccharide “inulin” to target the colon. In vitro drug release was measured in 0.1 M HCl for 2 h followed by phosphate buffer saline pH 6.8 to simulate gastric and intestinal fluids, respectively. Coated mini-tablets were exposed to fresh fecal samples of humans in order to simulate roughly colonic content. Several formulations were able to fully protect theophylline as a model drug up to 8 h in the upper GIT, but allowing for prolonged release kinetics in the colon. These very interesting colonic release profiles were related to the amount of the natural polysaccharide added into the system.
Graphical Abstract











Similar content being viewed by others
Data Availability
All data generated and/or analyzed during this study are included in this published article.
References
Tran PHL, Tran TTD. Current film coating designs for colon-targeted oral delivery. Curr Med Chem. 2021;28:1957–69.
Jones RGA, Martino A. Targeted localized use of therapeutic antibodies: a review of non-systemic, topical and oral applications. Crit Rev Biotechnol. 2016;36:506–20.
Vass P, Démuth B, Hirsch E, Nagy B, Andersen SK, Vigh T, et al. Drying technology strategies for colon-targeted oral delivery of biopharmaceuticals. J Control Release. 2019;296:162–78.
Truong-Le V, Lovalenti PM, Abdul-Fattah AM. Stabilization challenges and formulation strategies associated with oral biologic drug delivery systems. Adv Drug Deliv Rev. 2015;93:95–108.
Philip AK, Philip B. Colon targeted drug delivery systems: a review on primary and novel approaches. Oman Med J. 2010;25:79–87.
Awad A, Madla CM, McCoubrey LE, Ferraro F, Francesca KHG, Buanz A, et al. Clinical translation of advanced colonic drug delivery technologies. Adv Drug Deliv Rev. 2022;181: 114076.
Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet Lond Engl. 2017;389:1756–70.
Barberio B, Segal JP, Quraishi MN, Black CJ, Savarino EV, Ford AC. Efficacy of oral, topical, or combined oral and topical 5-aminosalicylates, in ulcerative colitis: systematic review and network meta-analysis. J Crohns Colitis. 2021;15:1184–96.
Gallo G, Kotze PG, Spinelli A. Surgery in ulcerative colitis: when? How? Best Pract Res Clin Gastroenterol. 2018;32–33:71–8.
Sousa T, Paterson R, Moore V, Carlsson A, Abrahamsson B, Basit AW. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int J Pharm. 2008;363:1–25.
Alqahtani MS, Kazi M, Alsenaidy MA, Ahmad MZ. Advances in Oral Drug Delivery. Front Pharmacol. 2021;12: 618411.
Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29:1035–41.
Koziolek M, Grimm M, Becker D, Iordanov V, Zou H, Shimizu J, et al. Investigation of pH and temperature profiles in the GI tract of fasted human subjects using the Intellicap® system. J Pharm Sci. 2015;104:2855–63.
Nugent SG, Kumar D, Rampton DS, Evans DF. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut. 2001;48:571–7.
Sasaki Y, Hada R, Nakajima H, Fukuda S, Munakata A. Improved localizing method of radiopill in measurement of entire gastrointestinal pH profiles: colonic luminal pH in normal subjects and patients with Crohn’s disease. Am J Gastroenterol. 1997;92:114–8.
Yuan Y, He N, Dong L, Guo Q, Zhang X, Li B, et al. Multiscale shellac-based delivery systems: from macro- to nanoscale. ACS Nano. 2021;15:18794–821.
Zhang C, Chen Z, He Y, Xian J, Luo R, Zheng C, et al. Oral colon-targeting core-shell microparticles loading curcumin for enhanced ulcerative colitis alleviating efficacy. Chin Med. 2021;16:92.
Panchapornpon D, Limmatvapirat C, Nuntanid J, Luangtana-Anan M, Sriamornsak P, Puttipipatkhachorn S, et al. Development of shellac from source available in Thailand as an alternative polymer for postharvest treatment. Thai J Agric Sc. 2011;44(5):224–9.
Chen Q, Zhang H, Zheng H, Sun Y. Solubility of lac resin in aqueous ammonia and physico-chemical properties. Food Sci. 2013;34(23):77–82.
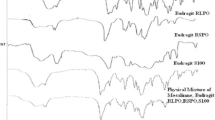
Al-Gousous J, Penning M, Langguth P. Molecular insights into shellac film coats from different aqueous shellac salt solutions and effect on disintegration of enteric-coated soft gelatin capsules. Int J Pharm. 2015;484:283–91.
Yan G, Cao Z, Devine D, Penning M, Gately N. Physical properties of shellac material used for hot melt extrusion with potential application in the pharmaceutical industry. Polym. 2021;13(21):3723.
Specht F, Saugestad M, Waaler T, Müller B. The application of shellac as an acidic polymer for enteric coating. Pharm Tech Eur. 1998;10(9):20–8.
Farag Y, Leopold CS. Physicochemical properties of various shellac types. Dissolution Technol. 2009;16:33–9.
Limmatvapirat S, Limmatvapirat C, Puttipipatkhachorn S, Nuntanid J, Luangtana-Anan M, et al. Enhanced enteric properties and stability of shellac films through composite salts formation. Eur J Pharmaceutics and Biopharmaceutics. 2007;67:690–8.
Thombare N, Kumar S, Kumari U, Sakare P, Yogi RK, Prasad N, et al. Shellac as a multifunctional biopolymer: a review on properties, applications and future potential. Int J Biol Macromol. 2022;215:203–23.
Habashy R, Khoder M, Zhang S, Pereira B, Bohus M, Tzu-Wenig Wang J, et al. An innovative wax-based enteric coating for pharmaceutical and nutraceutical oral products. Int J Pharm. 2020;591: 119935.
Data Sheet SWANLAC ASL 10. http://www.afsuter.com. https://www.afsuter.com/product/aqueous-shellac-solution (accessed 13th march 2023).
Theophylline water solubility at 25°C. http://www.pubchem.com. Available via Theophylline water solubility (accessed 13 October 2022).
Karrout Y, Neut C, Wils D, Siepmann F, Deremaux L, Flament MP, et al. Novel polymeric film coatings for colon targeting: drug release from coated pellets. Eur J Pharm Sci. 2009;37:427–33.
Karrout Y, Neut C, Wils D, Siepmann F, Deremaux L, Flament MP, et al. Peas starch-based film coatings for site-specific drug delivery to the colon. J Appl Polym Sci. 2011;119:1176–84.
Karrout Y, Neut C, Siepmann F, Wils D, Ravaux P, Deremaux L, et al. Enzymatically degraded Eurylon 6 HP-PG: ethylcellulose film coatings for colon targeting in inflammatory bowel disease patients. J Pharm Pharmacol. 2010;62:1676–84.
Karrout Y, Neut C, Wils D, Siepmann F, Deremaux L, Dubreuil L, et al. Colon targeting with bacteria-sensitive films adapted to the disease state. Eur J Pharm Biopharm. 2009;73:74–81.
Benzine Y, Siepmann F, Neut C, Danede F, Willart JF, Siepmann J, et al. Hot melt extruded polysaccharide blends for controlled drug delivery. J Drug Deliv Sci Technol. 2019;54: 101317.
Levingstone TJ, Herbaj S, Dunne NJ. Calcium phosphate nanoparticles for therapeutic applications in bone regeneration. Nanomater Basel Switz. 2019;9:E1570.
Rathbone MJ, Hadgraft J, Roberts MS, Lane ME. Modified-release drug delivery technology, Volume 2. 2nd Edition. CRC Press; 2013. Available from https://doi.org/10.3109/9781420045260
Hamedelniel EI, Bajdik J, Pintye-Hódi K. Optimization of preparation of matrix pellets containing ethylcellulose. Chem Eng Process Process Intensif. 2010;49:120–4.
Shah N, Sharma OP, Mehta T, Amin A. Design of experiment approach for formulating multi-unit colon-targeted drug delivery system: in vitro and in vivo studies. Drug Dev Ind Pharm. 2016;42:825–35.
Benzine Y, Siepmann F, Neut C, Danede F, Willart JF, Siepmann J, et al. Injection-molded capsule bodies and caps based on polymer blends for controlled drug delivery. Eur J Pharm Biopharm. 2021;168:1–14.
Esseku F, Adeyeye MC. Bacteria and pH-sensitive polysaccharide-polymer films for colon targeted delivery. Crit Rev Ther Drug Carrier Syst. 2011;28:395–445.
Haaser M, Karrout Y, Velghe C, Cuppok Y, Gordon KC, Pepper M, et al. Application of terahertz pulsed imaging to analyse film coating characteristics of sustained-release coated pellets. Int J Pharm. 2013;457:521–6.
Stillhart C, Vučićević K, Augustijns P, Basit AW, Batchelor H, Flanagan TR, et al. Impact of gastrointestinal physiology on drug absorption in special populations-An UNGAP review. Eur J Pharm Sci. 2020;147: 105280.
Ravi V, Siddaramaiah, Pramod Kumar TM. Influence of natural polymer coating on novel colon targeting drug delivery system. J Mater Sci Mater Med. 2008;19:2131–6.
Roda A, Simoni P, Magliulo M, Nanni P, Baraldini M, Roda G, et al. A new oral formulation for the release of sodium butyrate in the ileocecal region and colon. World J Gastroenterol. 2007;13:1079–84.
Farag Y, Leopold CS. Investigation of drug release from pellets coated with different shellac types. Drug Dev Ind Pharm. 2011;37:193–200.
Maghrabia AE, Boughdady MF, Meshali MM. New perspective enteric-coated tablet dosage form for oral administration of ceftriaxone: in vitro and in vivo assessments. AAPS PharmSciTech. 2019;20:306.
Wilding IR, Kenyon CJ, Hooper G. Gastrointestinal spread of oral prolonged-release mesalazine microgranules (Pentasa) dosed as either tablets or sachet. Aliment Pharmacol Ther. 2000;14:163–9.
Schellekens RCA, Stuurman FE, van der Weert FHJ, Kosterink JGW, Frijlinket HW. A novel dissolution method relevant to intestinal release behaviour and its application in the evaluation of modified release mesalazine products. Eur J Pharm Sci. 2007;30:15–20.
SSB® Pharma - Shellac for pharmaceutical applications: Available from SSB® Pharma: Shellac USP/Ph. Eur. http://www.elementoorganika.ru/files/SSB%20Pharma.pdf.
Buch K, Penning M, Wächtersbach E, Maskos M, Langguth P. Investigation of various shellac grades: additional analysis for identity. Drug Dev Ind Pharm. 2009;35:694–703.
Theismann E-M, Keppler JK, Knipp J-R, Fangmann D, Appel E, Gorb SN, et al. Adjustment of triple shellac coating for precise release of bioactive substances with different physico-chemical properties in the ileocolonic region. Int J Pharm. 2019;564:472–84.
Patel A, Heussen P, Hazekamp J, Velikov KP. Stabilisation and controlled release of silibinin from pH responsive shellac-colloidal particles. Soft Matter. 2011;7:8549.
Limmatvapirat S, Limmatvapirat C, Luangtana-Anan M, Nunthanid J, Oguchi T, Tozuka Y, et al. Modification of physicochemical and mechanical properties of shellac by partial hydrolysis. Int J Pharm. 2004;278:41–9.
Yuan Y, He N, Xue Q, Guo Q, Dong L, Haruna MH, et al. Shellac: A promising natural polymer in the food industry. Trends Food Sci Technol. 2021;109:139–53.
Kanwal S, Aliya S, Xin Y. Anti-obesity effect of Dictyophora indusiata mushroom polysaccharide (DIP) in high fat diet-induced obesity via regulating inflammatory cascades and intestinal microbiome. Front Endocrinol. 2020;11: 558874.
Mensink MA, Frijlink HW, Van Der Voort Maarschalk K, Hinrichs WLJ. Inulin, a flexible oligosaccharide. II: Review of its pharmaceutical applications. Carbohydr Polym. 2015;134:418–28.
Afinjuomo F, Abdella S, Youssef SH, Song Y, Garg S. Inulin and its application in drug delivery. Pharm Basel Switz. 2021;14:855.
López-Molina D, Navarro-Martínez MD, Rojas-Melgarejo F, Hiner ANP, Chazarra S, Rodriguez-Lopez J. Molecular properties and prebiotic effect of inulin obtained from artichoke (Cynara scolymus L.). Phytochemistry. 2005;66:1476–84.
Acknowledgements
The authors are deeply indebted to Prof. Pierre Desreumaux, Department of Gastroenterology CHU Lille, for the continuous support and advice. We would like to thank faithfully Mr. Rahul Mourya from A.F. Suter & Co Company in the UK for his generous supply of Shellac ASL 10 for film coatings. We are also deeply grateful to Dr. Tobias Hess from Chemische Fabrik Budenheim, in Germany, for his valuable supply of placebo mini-tablets.
Funding
M. Samuel Strich has received a salary from the university hospital of Lille (CHU: Centre Hospitalier Universitaire de Lille) to support his PhD within the framework of the hospital residency “IPR: Innovation Pharmaceutique et Recherche.” The authors are very grateful for this support. Sources of study funding have been from former industry research projects and University allocation of fund.
Author information
Authors and Affiliations
Contributions
S. Strich methodology; validation; investigation; visualization; conceptualization; writing—original draft—review and editing.
H. Azehaf review and editing; visualization
C. Neut methodology; validation; investigation; writing—review and editing.
Y. Lellouche-Jacob methodology; visualization; investigation.
N. Medkour methodology; visualization; investigation.
M. Penning methodology; validation; investigation; writing—review and editing
Y. Karrout conceptualization; methodology; resources; writing—original draft—review and editing; visualization; project administration; funding acquisition; supervision
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Strich, S., Azehaf, H., Neut, C. et al. Film Coatings Based on Aqueous Shellac Ammonium Salt “Swanlac® ASL 10” and Inulin for Colon Targeting. AAPS PharmSciTech 24, 205 (2023). https://doi.org/10.1208/s12249-023-02652-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02652-2