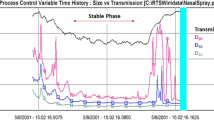
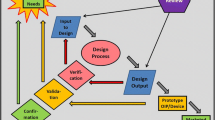
Abstract
Multiple sources must be consulted to determine the most appropriate procedures for the laboratory-based performance evaluation of aqueous oral inhaled products (OIPs) for the primary measures, dose uniformity/delivery, and aerodynamic particle (droplet) size distribution (APSD). These sources have been developed at different times, mainly in Europe and North America, during the past 25 years by diverse organizations, including pharmacopeial chapter/monograph development committees, regulatory agencies, and national and international standards bodies. As a result, there is a lack of consistency across all the recommendations, with the potential to cause confusion to those developing performance test methods. We have reviewed key methodological aspects of source guidance documents identified by a survey of the pertinent literature and evaluated the underlying evidence supporting their recommendations for the evaluation of these performance measures. We have also subsequently developed a consistent series of solutions to guide those faced with the various associated challenges when developing OIP performance testing methods for oral aqueous inhaled products.







Similar content being viewed by others
References
VanOort M. In vitro testing of dry powder inhalers. Aerosol Sci Technol. 1995;22(4):364–73.
Mitchell JP, Stein SW, Doub W, Goodey AP, Christopher JD, Patel RB, et al. Determination of passive dry powder inhaler aerodynamic particle size distribution by multi-stage cascade impactor: International Pharmaceutical Aerosol Consortium on Regulation & Science (IPAC-RS) recommendations to support both product quality control and clinical programs. AAPS PharmSciTech. 2019;20 article 206.
United States Pharmacopeial Convention. United States Pharmacopeia. Chapter <601>: Inhalation and nasal drug products: aerosols, sprays, and powders – performance quality tests. Rockwell, MD, USA. https://uspnf.com/uspnf/ (visited April 18, 2022).
European Directorate for the Quality of Medicines and Healthcare (EDQM). Strasbourg, France. Monograph 2.9.18: preparations for inhalation: aerodynamic assessment of fine particles. https://edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition (visited April 18, 2022).
Mitchell JP, Nagel MW. Cascade impactors for the size characterization of aerosols from medical inhalers: their use and limitations. J Aerosol Med. 2003;16(4):341–77.
ISO 27427:2013: anaesthetic and respiratory equipment - nebulizing systems and components. International Standards Organization, Geneva, Switzerland. https://www.iso.org/standard/59482.html (visited April 18, 2012).
Nerbrink O, Mitchell JP. Comparison of ISO standards for device performance: 20072 and 27427: a critical appraisal. J Aerosol Med Pulmon Drug Deliv. 2012;25(4):209–16.
Carvalho TC, McConville JT. The function and performance of aqueous aerosol devices for inhalation therapy. J Pharm Pharmacol. 2016;68:556–78.
Pritchard JN, Nazarzadeh E. Next generation nebulized therapy: opportunities for new treatments and devices. Respiratory Drug Delivery 2022, p. 103–112. ISBN 978–1–7396821–0–1. https://www.rddonline.com/rdd/rdd.php?id=21&sid=3. visited, April 29, 2022.
Hochrainer D, Hölz H, Kreher C, Scaffidi L, Spallek M, Wachtel H. Comparison of the aerosol velocity and spray duration of Respimat®Soft Mist™ Inhaler and pressurized metered dose inhalers. J Aerosol Med. 2005;18(3):273–82.
Wachtel H, Kattenbeck S, Dunne S, Disse B. The Respimat® development story: patient-centered innovation. Pulm Ther. 2017;3:19–30.
Nerbrink O. Nebulizers: past to present platforms and future possibilities. Inhalation. 2016;10(5):9–14.
Raabe OG, Wong TM, Wong GB, Roxburgh JW, Piper SD, Lee JI. Continuous nebulization therapy for asthma with aerosols of ß-2 agonists. Ann Allergy Asthma Immunol. 1998;80(6):499–508.
Mitchell JP, Suggett JA. Developing ways to evaluate in the laboratory how inhalation devices will be used by patients and caregivers: the need for clinically appropriate testing. AAPS PharmSciTech. 2014;15(5):1275–91.
Chatburn RL, McPeck M. A new system for understanding nebulizer performance. Respir Care. 2007;52(8):1037–50.
Dhand R. Nebulizers that use a vibrating mesh or plate with multiple apertures to generate aerosol. Respir Care. 2002;47(12):1406–16.
Lass JS, Sant A, Knoch M. New advances in aerosolized drug delivery: vibrating membrane nebuliser technology. Expert Opin Drug Deliv. 2006;3(5):693–702.
Gowda AA, Cuccia AD, Smaldone GC. Reliability of vibrating mesh technology. Respir Care. 2017;62(1):65–9.
Tseng H-Y, Lin H-L, Chiang H-S. In vitro evaluation of aerosol delivery by hand-held mesh nebulizers in an adult spontaneous breathing lung model. J Aerosol Med Pulmon Drug Deliv. 2022;35(2):83–90.
Vecellio L. The mesh nebuliser: a recent technical innovation for aerosol delivery. Breathe. 2006;2(3):253–60.
Flament MP, Leterme P, Gayot A. Study of the technological parameters of ultrasonic nebulization. Drug Dev Ind Pharm. 2001;27(7):643–9.
Phipps PR, Gonda I. Droplets produced by medical nebulizers: some factors affecting their size and solute concentration. Chest. 1990;97(6):1327–32.
Ari A. Jet, ultrasonic, and mesh nebulizers: an evaluation of nebulizers for better clinical outcomes. Eurasian J Pulmonol. 2014;16:1–7.
Niven RW, Ip A, Mittelman S, Prestrelski SJ, Arakawa T. Some factors associated with the ultrasonic nebulization of proteins. Pharm Res. 1995;12(1):53–9.
Rajapaska A, Qi A, Yeo LY, Coppel R, Friend JR. Enabling practical surface acoustic wave nebulizer drug delivery via amplitude modulation. Lab Chip. 2014;14(11):1858–65.
Yousefi M, Pourmehran O, Gorji-Bandpy, Inthavong K, Yeo L, Tu J. CFD simulation of aerosol delivery to a human lung via surface acoustic wave nebulization. Biomech Model Mechanobiol. 2017;16: 2035–2050.
Friend J, Yeo LY. Microscale acoustofluidics: microfluidics driven via acoustics and ultrasonics. Rev Mod Phys. 2011;83:647–704.
Yeo LY, Friend JR. Ultrafast microfluidics using surface acoustic waves. Biomicrofluidics 2009;3:article 012002.
Brenker J, Al-Mazi JG, Le A, Pal S, Ong HX, Traini D, et al. Microfluidic aerosol generation: development of a next generation hand-held device for aerosol drug delivery. Respiratory Drug Delivery 2022, p. 277–80. ISBN 978–1–7396821–0–1. https://www.rddonline.com/rdd/rdd.php?id=21&sid=3. visited, April 29, 2022.
McCormack P, Southern KW, McNamara PS. New nebulizer technology to monitor adherence and nebulizer performance in cystic fibrosis. J Aerosol Med Pulmon Deliv. 2012;25(6):307–9.
Pritchard JN, Hatley RHM, Denyer J, von Hollen D. Mesh nebulizers have become the first choice for new nebulized pharmaceutical drug developments. Ther Deliv. 2018;9(2):121–36.
Kesten S, Israel E, Li G, Mitchell J, Wise R, Stern T. Development of a novel digital breath-activated inhaler: initial particle size characterization and clinical testing. Pulm Pharmacol Ther. 2018;53(2):7–32.
Dhand R. Intelligent nebulizers in the age of the internet: the I-neb® adaptive aerosol delivery (AAD) system. J Aerosol Med Pulmon Deliv. 2010;23Suppl.1: iii-v.
Nikander K, Denyer J. Adaptive aerosol delivery (AAD) technology. In: Rathbone MJ, editor. Modified Release Drug Delivery Technology, 2nd ed. Informa Healthcare USA, Inc., NY, USA. 2008. p. 603–12.
Fischer A, Stegemann J, Scheuch G, Siekmeier R. Novel devices for individualized controlled inhalation can optimize aerosol therapy in efficacy, patient care and power of clinical trials. Eur J Med Res. 2009;14(Suppl. IV):71–7.
United States Food and Drug Administration. Draft guidance on budesonide. September 2012. Silver Spring, MD, USA. https://www.accessdata.fda.gov/drugsatfda_docs/psg/Budesonide_Inhalation_Sus_20929_RC_09-12.pdf. (Visited April 28, 2022).
Boe J, Dennis JH, O’Driscoll BR, Bauer TT, Carone M, Dautzenberg B, et al. European Respiratory Society Guidelines on the use of nebulizers. Eur Respir J. 2001;18(1):228–42.
United States Pharmacopeial Convention. Chapter <1601> Products for nebulization. In: United States Pharmacopeia. Rockwell, MD, USA. https://uspnf.com/uspnf/ (visited April 19, 2022).
European Directorate for the Quality of Medicines and Healthcare (EDQM). Monograph 2.9.44. Preparations for nebulization. In: European Pharmacopoeia. EDQM: Strasbourg, France. https://edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition (visited April 19, 2022).
United States Food and Drug Administration. Draft guidance on tiotropium bromide. Silver Spring, MD, USA. November 2020. https://www.accessdata.fda.gov/drugsatfda_docs/psg/Tiotropium%20bromide_inhalation%20powder_NDA%20021395_RC10-17.pdf. (Visited April 28, 2022).
United States Food and Drug Administration. Draft guidance on albuterol sulfate; ipratropium bromide. Silver Spring, MD, USA. August 2021. https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_021747.pdf. (Visited April 28, 2022).
United States Food and Drug Administration. Draft guidance on Olodaterol hydrochloride. Silver Spring, MD, USA. August 2021. https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_203108.pdf. (Visited April 28, 2022).
United States Food and Drug Administration. Draft guidance on olodaterol hydrochloride; tiotropium bromide. Silver Spring, MD, USA. July 2021. https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_206756.pdf. (Visited April 28, 2022).
United States Food and Drug Administration: Metered dose inhaler (MDI) and dry powder inhaler (DPI) drug products: quality considerations. https://fda.gov/regulatoryinformation/search-fda-guidance-documents/metered-doseinhaler-mdi-and-drypowderinhaler-dpi-drug-products-quality-considerations (visited on April 19, 2022).
European Directorate for the Quality of Medicines and Healthcare (EDQM). Monograph 0671. Preparations for inhalation: Inhalanda. In: European Pharmacopoeia. EDQM: Strasbourg, France. https://edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition (last visited April 19, 2022).
United States Food and Drug Administration. Jurisdictional update: metered dose inhalers, spacers and other accessories. 2020. www.fda.gov/combination-products/jurisdictional-updates/jurisdictional-update-metered-dose-inhalers-spacers-and-other-accessories (Visited April 19, 2022).
European Medicines Agency (EMA): European Medicines Agency (EMA): guideline on the pharmaceutical quality of inhalation and nasal products. London, UK. EMEA/ CHMP/QWP/49313/2005 Corr.654 2006. 2006. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003568.pdf. (Visited on April 19, 2022.)
Health Canada (HC): Guidance for industry: pharmaceutical quality of inhalation and nasal products. Document 06–106624–547. Ottawa, Canada. 2006. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/chemical-entity-products-quality/guidance-industry-pharmaceutical-quality-inhalation-nasal-products.html. (Visited on April 19, 2022.)
United States Food and Drug Administration. Reviewer guidance for nebulizers, metered dose inhalers, spacers, and actuators. Silver Spring, MD, USA. October 1993. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reviewer-guidance-nebulizers-metered-dose-inhalers-spacers-and-actuators. (Visited April 28, 2022).
Stein SW, Thiel CG. The history of therapeutic aerosols: a chronological review. J Aerosol Med Pulmon Deliv. 2017;30(1):20–41.
Chandel A, Goyal AK, Ghosh G, Rath G. Recent advances in aerosolized drug delivery. Biomed Pharmacother. 2019;112 article 108601.
Hess DR, MacIntyre NR, Mishoe SC, Galvin WF, Adams AB, Saposnick AB. Respiratory care: principles & practice. Philadelphia: WB Saunders; 2002. p. 643–9.
Dalby RN, Eicher J, Zierenberg J. Development of Respimat® Soft Mist™ Inhaler and its clinical utility in respiratory disorders. Med Dev Evid Res. 2011;4:145–55.
Mehri R, Alatrash A, Matida EA, Fiorenza F. Respimat® soft mist inhaler (SMI) in-vitro aerosol delivery with the ODAPT™ adapter and facemask. Can J Respir Crit Care Sleep Med. 2019;5(3):1–10.
Stein S. Aiming for a moving target: challenges associated with impactor measurement of MDI aerosols. Int J Pharm. 2008;355(1–2):53–61.
Mitchell JP, Nagel MW, Avvakoumova V, MacKay H, Ali R. The abbreviated impactor measurement (AIM) concept: part 2—influence of evaporation of a volatile component— evaluation with a “droplet producing” pressurized metered dose inhaler (pMDI)-based formulation containing ethanol as co-solvent. AAPS PharmSciTech. 2009;10:252–7.
Mitchell JP, Nagel MW, Nichols S, Nerbrink O. Laser diffractometry as a technique for the rapid assessment of aerosol particle size from inhalers. J Aerosol Med. 2006;19(4):409–33.
Hatley RHM, Byrne SM. Variability in delivered dose and respirable delivered dose from nebulizers: are current regulatory testing guidelines sufficient to produce meaningful information? Med Dev Evid Res. 2017;10:17–28.
Rau JL, Ari A, Restrepo RD. Performance comparison of nebulizer designs: constant-output, breath-enhanced, and dosimetric. Respir Care. 2004;49(2):174–9.
Ari A, de Andrade AD, Sheard M, Al Hamad B, Fink JB. Performance comparisons of jet and mesh nebulizers using different interfaces in simulated spontaneously breathing adults and children. J Aerosol Med Pulmon Drug Deliv. 2015;28(4):281–9.
Roth AP, Lange CF, Finlay WH. The effect of breathing pattern on nebulizer drug delivery. J Aerosol Med. 2003;16(3):325–9.
Kwong WTJ, Ho SL, Coates AL. Comparison of nebulized particle size distribution with Malvern laser diffraction analyzer versus Andersen cascade impactor and low-flow Marple personal cascade impactor. J Aerosol Med. 2000;13(4):304–14.
United States Pharmacopeial Convention. Chapter <429> Light diffraction measurement of particle size. In: United States Pharmacopeia. Rockwell, MD, USA. https://uspnf.com/uspnf/ (visited May 18, 2022).
Holmes CE, Kippax PG, Newell HE, Southall JP, Ward DJ. Simultaneous analysis of respirable aerosols via laser diffraction and cascade impaction. In: Drug Delivery to the Lungs-13. Aerosol Society, London, UK, 2001, p. 58–61.
Clark A, Borgström L. In vitro testing of pharmaceutical aerosols and predicting lung deposition from in vitro measurements. In: Bisgaard H, O’Callaghan C, Smaldone GC, editors. Drug Delivery to the Lung. Marcel Dekker, NY, USA, 2002; p.105–42.
European Directorate for the Quality of Medicines and Healthcare (EDQM). Monograph 2.9.31. Particle size analysis by laser light diffraction. In: European Pharmacopoeia. EDQM: Strasbourg, France. https://edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition. (Visited May 18, 2022).
ISO 13320–1:2020: Particle size analysis – laser diffraction methods. International Standards Organization, Geneva, Switzerland. https://www.iso.org/standard/59482.html (visited April 18, 2012).
Malvern Instruments. Introducing the Malvern Spraytec: accurate particle sizing of aerosols and sprays. Malvern Panalytical, Malvern, UK. 2022. https://www.malvernpanalytical.com/en/assets/MRK0690-04_spraytec_2016_LRA4_9page_tcm50-17212.pdf. (Visited May 18, 2022).
Song X, Hu J, Zhan S, Zhang R, Tan W. Effects of temperature and humidity on laser diffraction measurements to jet nebulizer and comparison with NGI. AAPS PharmSciTech. 2016;17(2):380–8.
Marple VA, Olson BA, Miller NC. The role of inertial particle collectors in evaluating pharmaceutical aerosol systems. J Aerosol Med. 1998;11S1:S139–53.
Vecellio None L, Grimbert D, Bequemin LH, Boissinet O, Le Pape A, Lemarié E, et al. Validation of laser diffraction method as a substitute for cascade impaction in the European project for a nebulizer standard. J Aerosol Med. 2001;14(1):107–14.
Ziegler J, Wachtel H. Comparison of cascade impaction and laser diffraction for particle size distribution measurements. J Aerosol Med. 2005;18(3):311–24.
Finlay WH, Stapleton KW. Undersizing of droplets from a vented nebulizer caused by aerosol heating during transit through an Anderson impactor. J Aerosol Sci. 1999;30(1):105–9.
Dennis JH. Nebulizer efficiency: modeling versus in vitro testing. Respir Care. 2007;52(8):984–8.
Schuschnig U, Heine B, Knoch M. How cold is cold enough? Refrigeration of the next-generation impactor to prevent aerosol undersizing. J Aerosol Med Pulmon Drug Deliv. 2022;35(1):25–31.
Roth C, Gebhart W. Aqueous droplet sizing by inertial classification. Part Part Syst Charact. 1996;13:192–5.
Marple VA, Olson BA, Santhanakrishnan K, Roberts DL, Mitchell JP, Hudson-Curtis BL. Next generation pharmaceutical impactor: a new impactor for pharmaceutical inhaler testing. Part III. Extension of archival calibration to 15 L/min. J Aerosol Med. 2004;17(4):335–43.
Acknowledgements
The authors wish to acknowledge support and advice from colleagues working within individual member companies of IPAC-RS who are involved with the day-to-day performance testing of aqueous inhalers for oral use.
Author information
Authors and Affiliations
Contributions
JPM and WHD led the preparation of the manuscript and undertook surveys of the pertinent literature; CJG and BBL provided perspective from a high throughput testing lab, including the two specific examples to illustrate challenges in adhering to the compendial methodology when testing specific nebulizers; all authors contributed to the discussion surrounding content; all authors reviewed draft versions of the manuscript, contributing to the technical content.
Corresponding author
Ethics declarations
Conflict of Interest
Apart from MC, who is the CEO of a company that manufactures inhaler test equipment, there are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mitchell, J.P., Carter, I., Christopher, J.D. et al. Good Practices for the Laboratory Performance Testing of Aqueous Oral Inhaled Products (OIPs): an Assessment from the International Pharmaceutical Aerosol Consortium on Regulation and Science (IPAC-RS). AAPS PharmSciTech 24, 73 (2023). https://doi.org/10.1208/s12249-023-02528-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02528-5