Abstract
Pollution is a worldwide environmental risk. Arsenic (As) is an environmental pollutant with a major health concern due to its toxic effects on multiple body organs, including the brain. Humans are exposed to As through eating contaminated food and water or via skin contact. Salix species (willow) are plants with medicinal efficacy. Salix subserrata Willd bark extract-loaded chitosan nanoparticles (SBE.CNPs) was formulated, characterized, and evaluated against As-induced neurotoxicity. The stem bark was selected for nanoparticle formulation based on HPLC–PDA-ESI–MS/MS profiling and in vitro antioxidant assessment using free radical scavenging activity. SBE.CNPs demonstrated an average un-hydrated diameter of 193.4 ± 24.5 nm and zeta potential of + 39.6 ± 0.4 mV with an encapsulation efficiency of 83.7 ± 4.3%. Compared to As-intoxicated rats, SBE.CNP-treated rats exhibited anxiolytic activity and memory-boosting as evidenced in open field test, light–dark activity box, and Y-maze. Also, it increased the antioxidant biomarkers, including superoxide dismutase and glutathione peroxidase associated with reducing the malondialdehyde levels and apoptotic activity. Besides this, SBE.CNPs maintained the brain architecture and downregulated both nuclear factor-kappa B and heme oxygenase-1 expression. These results suggest that SBE.CNP administration showed promising potent neuroprotective and antioxidative efficiencies against arsenic-induced oxidative threats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Occupational and environmental exposure to inorganic arsenic (As) have a detrimental effect on health globally [1]. Contamination of the food and the underground water, inhalation, and dermal contact is the major sources of As exposure [1, 2]. According to World Health Organization (WHO), the highest level for arsenic contamination in drinking water is 10 ppb (parts per billion; 10 ppb = 10 μg/L) [3]. As has a toxic effects on multiple body organs, including the brain, and these effects could be attributed to its ability to cross the blood–brain barrier and accumulate in the brain even at a low dose leading to neurotoxcity. Also, the vulnerability of the brain to oxidative damage due to its high metabolic rate [4, 5].
One of the mechanisms by which arsenic leads to neurotoxcity is due to oxidative stress with exccessive generation of reactive oxygen species (ROS) such as superoxides and hydrogen peroxide [6, 7]. These ROS consequently lead to activation of many transcription factors, including, nuclear factor kappa B (NF-kB) that results in production of pro-inflammatory cytokines and cytotoxic genes [7]. NF-κB plays a critical role in regulating the survival, activation, and differentiation of innate immune cells and inflammatory T cells [8]. Moreover, NF-kB upregulates heme oxygenase-1 (HO-1) gene expression that stimulates the expression of pro-inflammatory genes [9].
The curative potential of many plant extracts has received considerable critical attention. One of these medicinal plants is belonging to the Salicaceae family which includes Salix (willow) that is common in Northern temperate regions. Different phytoconstituents were isolated/and or identified from genus Salix such as flavonoids, phenolic and non-phenolic glycosides, organic acids, and their derivatives, sterols, and terpenes, volatiles, and fatty acids [10]. Furthermore, Salix species, including Salix subserrata Willd, have been reported to possess analgesic, anti-inflammatory, antioxidant, anticancer, cytotoxic, antimicrobial, anti-obesity, neuroprotective, and hepatoprotective activities [10,11,12].
Nanotechnology was utilized to increase the solubility and efficacy of natural plants. Chitosan is a widely used polymer in drug delivery compounds with an acceptable safety profile [13,14,15]. Although some research has been carried out on gold nanoparticle synthesis using the bark and leave extract of Salix alba [16, 17], no one reports the synthesis of nanoparticles using S. subserrata stem bark extract.
Therefore, in the present study, the chemical profiling of S. subserrata stem bark (SB), shoots (SS), and leaves (SL) was performed by LC/MS, followed by in vitro antioxidant activity using DPPH assay. Then, the chitosan nanoparticles (CNPs) were loaded with the extract of S. subserrata bark (SBE), characterized, and evaluated for As-induced neurotoxicity in Wistar rats.
Materials and Methods
Plant Material and Extraction
Stem bark, shoots, and leaves of Salix subserrata Willd (Salicaceae) were collected in March 2016 from the vicinity of Zagazig city (Sharkia governorate, Egypt). Plant identification was confirmed by Dr. H. Abdelbaset, Prof. of plant taxonomy, Faculty of Science, Zagazig University, Egypt. Voucher specimens (accession no. SSU-2) were deposited in the Department of Pharmacognosy, Faculty of Pharmacy, Zagazig University, Egypt. The dried plant materials (200 g each) were extracted twice with 80% aqueous methanol (2 × 1 L) at room temperature for 3 days. The obtained extracts were filtered, concentrated using a rotary evaporator at 40°C, and lyophilized to yield 31, 24, and 30 g of bark (SBE), shoots (SSE), and leaves (SLE), respectively.
HPLC–PDA-ESI–MS/MS Analysis
S. subserrata total extract of stem bark, shoots, and leaves (100 mg/mL) were prepared using HPLC analytical-grade methanol solvent, filtered using a membrane disc filter (0.2 m), then subjected to chemical profiling using high-performance liquid chromatography-photodiode array-electrospray ionization mass spectrometry (HPLC–PDA-MS/MS), where Thermofinigan (Thermo Electron Corporation, USA) coupled with an LCQ-Duo ion trap mass spectrometer with an ESI source (ThermoQuest) was used. A C18 reversed-phase column (Zorbax Eclipse XDBC18, rapid resolution, 4.6 × 150 mm, 3.5 µm, Agilent, USA) was used for separation. A mobile phase of water and acetonitrile (ACN) was applied from 5 to 30% ACN in 60 min and then was increased to 90% ACN in the next 60 min with a flow rate of 1 ml/min with a 1:1 split before the ESI source. The following conditions were operated in MS: capillary voltage (− 10 V), the source temperature was set at 200°C, and nitrogen was used as a sheath and auxiliary gas at a flow rate of 80 and 40 (arbitrary units), respectively. MS/MS fragmentation was recorded with a collision energy of 35% in a negative ion mode. The ions were detected in a full scan mode and a mass range of 50–2000 m/z. The machine was controlled using Xcalibur software (Xcaliburۛ 2.0.7, Thermo Scientific).
In Vitro Antioxidant Assessment
Free radical scavenger activity (RSA) of crude extracts of SBE, shoots (SSE), and leaves (SLE) were measured by 1,1-diphenyl-2-picryl-hydrazil (DPPH) assay as previously described [18]. Briefly, the violet color solution of 0.1 mM DPPH faded to yellow in the presence of antioxidant molecules. The intensity of these colors was detected spectrophotometrically at 517 nm using a plate reader (Tecan, USA) to quantify RSA % at different concentrations of extracts (50, 100, 150, 200, 300, 400 μg/mL) according to the following equation:
where A0 is the absorbance of the control, and A1 is the absorbance of the extract. Methanol was used as a blank.
Bark Extract-Loaded Chitosan Nanoparticle Preparation
Chitosan nanoparticles loaded with bark extract (SBE.CNPs) were synthesized using the ionic gelation technique [19]. Briefly, chitosan (Mw ~ 100:300 KDa, deacetylated degree 90%; ACROS ORGANICS®; UK) was dissolved in dilute acetic acid (2% v/v) overnight at room temperature to form a 2 mg/mL concentration solution, and pH was adjusted to 5 using 1 M NaOH. Sodium tripolyphosphate (TPP) was dissolved in water to reach a final concentration of 1 mg/mL. The next day, the SBE solution was prepared by dissolving in 95% ethanol at a concentration of 2 mg/mL. A specific volume of ethanolic solution of SBE (10 wt% of chitosan) was mixed with TPP. Next, a TPP and SBE solution mixture was added to chitosan to reach a final mass ratio of 3:1 (chitosan:TPP) and then left to stir for 45 min. Afterward, the NP solution was centrifuged at 12 000 × g for 20 min and washed with distilled water three times. After washing, the NP solution was frozen down at − 20°C for 4 h and then put in a lyophilizer for 48 h.
Bark Extract-Nanoparticles (SBE.NPs) Characterization
NP diameter was determined using scanning electron microscopy (SEM) (XL-30 ESEMFEG SEM, FEI Company, USA). NPs were mounted on carbon tape and sputter-coated with a thin layer of gold/palladium. The average diameters of 500 particles were determined from SEM images (n = 3) using image analysis software (ImageJ, National Institutes of Health, version 1.5a, ImageJ.nih.gov).
Dynamic light scattering, polydispersity index (PDI), and zeta potential analyses were performed to determine the hydrodynamic diameter, particle size distribution, and surface charge of hydrated NPs. Briefly, 1 mg/mL of SBE-CNPs in deionized water (diH2O) was prepared. After vertexing and sonication, samples were diluted at a 1:50 ratio in diH2O. One milliter was aliquoted to the cuvette for analysis (Zetasizer Nano ZS90, Malvern, UK).
To determine encapsulation efficiency (EE) as previously described [13], 1 mg/mL SBE-CNP solution was centrifuged at 16,000 × g for 30 min. The supernatants were transferred and the pellet was washed twice with ethanol to remove unbound SBE from the formulated NPs. The amount of SBE in all resulting supernatant was determined through the UV spectrum at an absorbance of 278 nm. SBE quantity was determined from a standard curve of known SBE concentrations. Encapsulation efficiency was calculated according to the following equation.
In vitro release was evaluated by gentle agitation of NPs in phosphate-buffered saline (pH 7.4) at 37°C. Samples were collected at different time points (1, 2, 4, 8, 24 h), and the amount of SBE released from the NPs was quantified as described above.
In Vivo Evaluation of SBE.CNPs Against As-Induced Neurotoxicity
Animals
Twenty-four male albino rats weighing 150 and 180 g were purchased from the breeding facility of the Faculty of Veterinary Medicine, Cairo University, Egypt. They were acclimatized for 1 week before starting the experimental study. The environmental conditions were optimum (temperature of 25 ± 2°C, relative humidity of 50 ± 5%, and 12-h dark:12-h light cycle). The rats were fed with a standard ration during the study and had free access to water. The experimental design was in accordance with ARRIVE guidelines, agreed by the Veterinary Institutional Animal Care and Use Committee (VET- IACUC; approval number: VetCU 8/03/2022/394), and in compliance with the AVMA Guidelines for the Euthanasia of Animals: 2013 Edition [20].
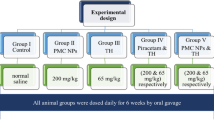
Experimental Design
Rats were randomly distributed into three treatment groups consisting of eight rats/group as follows:
-
Group I (control group): rats received distilled water through oral gavage for 2 weeks.
-
Group II (arsenic, As group): rats were treated with sodium arsenate (dissolved in distilled water, 20 mg/kg body weight) through oral gavage for 2 weeks.
-
Group III (As + SBE.CNPs): rats were concomitantly given sodium arsenate (20 mg/kg body weight) and SBE.CNPs (50 mg/kg) through oral gavage for 2 weeks.
The dosage of sodium arsenate and extract were selected according to previous literature [21]. The experimental design is illustrated in Fig. 1
Behavioral Testing
On the 15th to 17th day, the rats were transferred to the behavioral room to evaluate their anxiety-like behavior using an open field test and dark light activity box. Their cognitive abilities were also assessed using Y-maze according to previous studies [22, 23].
Blood and Tissue Sampling
After the last day of the behavioral test (day 18th), blood samples were collected from the eye’s inner canthus and centrifuged at 3000 rpm for 15 min to collect sera. Then, rats were euthanized by cervical dislocation, and brains were excised quickly, washed with cold saline, and preserved in either neutral buffered formalin (10%) for histopathological and immunohistochemical investigations or deep freezer (− 80°C) for subsequent biochemical analyses.
Biochemical Analysis
Determination of Superoxide Dismutase Activity
Superoxide dismutase (SOD) activity in the brain tissues was measured using colorimetric kits (Biodiagnostic Co, Cairo, Egypt) according to the manufacturer’s instructions. In this assay, SOD inhibits the reduction of nitro blue tetrazolium dye that can be measured at λmax 560 nm, and the change in the absorbance was recorded to determine its activity.
Assessment of Brain Glutathione Peroxidase Activity
The glutathione peroxidase (GSH-Px) activity was analyzed using colorimetric kits (Biodiagnostic Co, Cairo, Egypt) according to the manufacturer’s instructions. GSH-Px activity is accompanied by oxidation of nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) to NADP+ that can be quantified at λmax 340 nm. Brain homogenate was used to assess glutathione, glutathione reductase, NADPH, and hydrogen peroxide for measuring GSH-Px activity.
Measurement of Lipid Peroxidation
Malondialdehyde (MDA) as a lipid peroxidation marker was quantified in the brain tissue using a lipid peroxide colorimetric kit (Biodiagnostic Co, Cairo, Egypt) described by the manufacturer. Briefly, malondialdehyde content in brain tissue reacts with thiobarbituric acid to form thiobarbituric acid reactive products that can be quantified spectrophotometrically by measuring the absorbance of the solution at λmax 534 nm.
Estimation of DNA Fragmentation as an Apoptosis Hallmark Using TUNEL Assay
To study the extent of DNA fragmentation and apoptosis in the brain tissue, the brain cells were diced into 5 mm × 5 mm sections and fixed by immersion in phosphate-buffered saline (PBS) containing 4% paraformaldehyde for 24 h at 4°C. The fixed tissue was then embedded in Tissue Path (Curtin Matheson Scientific Inc.). Tissue Sects. (4 µm) were prepared using a microtome, and the TUNEL assay was performed on brain tissue following the manufacturer’s instructions provided by Bio vision (Milpitas, USA). Apoptosis was determined by counting the number of TUNEL-positive cells in 30 random microscopic fields. Images were acquired by fluorescence microscopy (IX61, Olympus, Japan). Cells were considered positive if they stained bright green.
Histopathological Analysis
After fixation of brain tissues for 24 h in 10% neutral buffered formalin, tissues were settled in paraffin. Five-micrometer tissues were cut into sagittal sections for hematoxylin and eosin (H&E) staining [24]. Histomorphometry evaluation was performed by Olympus BX43 light microscope linked with a digital camera. Finally, the histological analysis of different lesions, including neuronal degeneration, neurophagia, and gliosis, was graded separately for each lesion.
Concisely, the degeneration and neurophagia were assessed as follows: 0 = absent, 1 = focal distributed neurons (< 25%), 2 = focal distributed neurons (25–50%), 3 = focal distributed neurons (> 75), 4 = multifocal neurons (> 75), whereas the gliosis was scored along this way: 0 = absent, 1 = mild (< 25%), 2 = moderate (25–50%), and 3 = severe (> 75). The final score for each rat was calculated by summation of the total lesions score (4–5 fields/200 ×).
Immunohistochemistry Analysis
For detecting the expression of nuclear factor-kappa B (NF-ƘB) and heme oxygenase-1 (HO-1), the deparaffinized slides were rehydrated with alcohol, dipped in peroxidase solution, washed with PBS, and incubated with the polyclonal antibody of NF-ƘP or HO-1. Subsequently, the slides were immersed in PBS three times and kept with secondary antibody for 30 min. To promote the color reaction, 2 mL of DAB-chromgen-substrate was added for 15 min. Finally, a hematoxylin stain was applied for counterstaining. Positive results were calculated as area% using ImageJ software.
Statistical Analysis
Data were presented as mean ± SEM. Statistical analysis was performed using one-way analysis variance (ANOVA) followed by Tukey–Kramer post hoc test. However, histological damage scoring of different brain regions was performed using Kruskal–Wallis test, followed by Dunn’s Multiple Comparison Test. All statistical analyses were performed using GraphPad Prism software version 5 (ISI® software, USA).
Results
Chemical Profiling of S. subserrata Plant Organs
The total alcoholic extracts of SBE, SSE, and SLE were analyzed by HPLC–PDA-MS/MS in a negative ion mode because it is more sensitive than a positive one for the detection of different phenolic compounds [25]. Totally, 102 secondary metabolites were tentatively identified (10 compounds were previously isolated, while the others are first identified in S. subserrata). The identified metabolites are regarding different categories: proanthocyanidins (monomers, dimers, and trimers), phenolic acids, and their derivatives, salicinoids, flavonoids, cyclohexanediol glycosides, and fatty acids that are listed in Table I and illustrated by Fig. 2, 1SA-C, Fig. 2-6S, and ST1. The identification was based on the UV spectrum, MS2 fragmentation of the precursor ion, alongside neutral mass loss and characteristic fragmentation patterns for the given classes of compounds together with a comparison with the available literature. The compounds were ordered according to their relative retention time (RRt) to acetyl salicotrin. This is due to the retention of the same compound that exists in all extracts slightly different from one extract to another even under identical conditions; thus, it is important to select a compound that exists in all fractions and calculate the retention time of all eluted compounds relative to this selected compound.
In Vitro Antioxidant Assessment
The crude methanolic extracts (SBE, SSE, and SLE) exhibited potent antioxidant activity through their ability to scavenge free radicals in the DPPH assay while the SBE exerts the highest activity with IC50 = 16.8 µg/mL (the concentration which exhibited 50% scavenging for DPPH radicals) relative to SSE and SLE (Fig. 3a).
a Scavenging activity of Salix subserrata extracts against DPPH radical. Bark (SBE), leaf (SLE), and shoot (SSE); b SEM images of SBE.CNPs. Scale bar represents 200 nm. Images are representative of a minimum of 3 independent samples, with n > 500 NPs, and c cumulative release of SBE for 24 h. Data were represented as (mean ± SD)
SBE.NP Characterization
SBE.NPs demonstrated average unhydrated and hydrated diameters of 193.4 ± 24.5 nm and 216 ± 22.6 nm, respectively. SBE.CNPs showed PDI and zeta potential of 0.22 ± 0.03 and + 39.6 ± 0.4 mV, respectively. Loading experiments showed that chitosan highly encapsulated SBE with an encapsulation efficiency of 83.7 ± 4.3%. SBE release pattern demonstrated a burst release at the first hours of incubation, then gradually released until the end of the experiment at 44.4 ± 4.4% at 24 h (Fig. 3b, c).
Behavioral Parameters
As-intoxicated rats displayed a significantly increased anxiety-like behavior visualized by an increase in the time spent in the dark chamber of the light–dark activity (238 ± 21.65) box associated with a marked decrease in the time spent in the light chamber (62 ± 21.65) compared to controls (147.8 ± 64.59, 152.17 ± 64.59, respectively). Moreover, they exhibited less frequent entry to either the dark or light chambers (3.8 ± 0.7, and 3.67 ± 0.51, respectively). However, SBE.CNP-treated rats displayed a significant decrease in the time spent in the dark chamber associated with an increase in the time spent in the light chamber (140.17 ± 13.7, 159.83 ± 13.73, respectively). Also, they exhibited a more frequent entry to either the dark or light chambers (7.67 ± 1.63, 8.09 ± 0.6, respectively) compared to As-intoxicated rats (Fig. 4a–d).
a–d Effect of arsenic (As) and Salix subserrata bark extract-chitosan nanoparticle (SBE.CNPs) administration on the anxiety-like behaviour of rats in the light dark activity box. Data are expressed as mean ± SEM, one-way ANOVA followed by post hoc test Tukey test for eight rats in each group. *Significant from the control group and.$significant from As group. P < 0.05
Furthermore, As-intoxicated rats displayed a significant decrease in their locomotor and exploratory activities compared to controls expressed by a decrease in the number of crossing squares as well as the rearing episodes (35.67 ± 10.7, 7.17 ± 1.47, respectively) compared to controls (59.83 ± 6.55, 12.50 ± 3.2, respectively). Conversely, SBE.CNP-treated rats showed a substantial increase in the general locomotor and exploratory activities compared to As-intoxicated rats (75.67 ± 13.3, 18 ± 2.8, respectively) (Fig. 5a, b).
a–d Effect of arsenic (As) and Salix subserrata bark extract-chitosan nanoparticle (SBE.CNPs) administration on locomotion, exploration, and memory of rats in the open field test and Y-maze. a. Open field test: number of crossings, b. Open field test: rearing frequency, c. Y-maze test: number of arm entries, and d. Y-maze test: SAP% (spontaneous alternation percentage). Data are expressed as mean ± SEM, one-way ANOVA followed by post hoc test Tukey test for eight rats in each group. *Significant from the control group and.$significant from As group. P < 0.05
Concerning the cognitive abilities of As-intoxicated rats, it was markedly affected as expressed by a decrease in total arm entries and spontaneous alternation percentage (SAP) % (7.17 ± 1.47, 49.67 ± 4.7, respectively) compared to controls (10.17 ± 1.6, 64.17 ± 5.8, respectively). On the other hand, SBE.CNP-treated rats showed a substantial increase in total arm entries and SAP% compared to As-intoxicated rats (15.67 ± 3.3, 64.33 ± 6.37, respectively) (Fig. 5c, d).
Biochemical Parameters
Determination of SOD Activity
As-intoxicated rats exhibited significant decreases in the SOD activity (30.07 ± 4.66) compared to the control (66.83 ± 3.47, P ≤ 0.001) and SBE.CNP-treated rats (53.37 ± 4.90, P ≤ 0.01) (Fig. 6a).
Effect of arsenic (As) and Salix subserrata bark extract-chitosan nanoparticle (SBE.CNPs) administration on the oxidative stress of rats. a Superoxide dismutase (SOD). b Glutathione peroxidase (GSH-Px), and c malondialdehyde (MDA). Data are expressed as mean ± SEM, one-way ANOVA followed by post hoc test Tukey test for eight rats in each group. *Significant from the control group and.$significant from As group. P < 0.05
Assessment of Brain GSH-Px Activity
The brain tissue homogenate of As-intoxicated rats demonstrated a significant reduction of GSH-Px activity (13.70 ± 3.29, P ≤ 0.001) compared to control animals (39.55 ± 1.41). Additionally, SBE.CNP-treated rats showed a significant increase in GSH-Px activity (32.00 ± 2.92, P ≤ 0.001) compared to the arsenic group (Fig. 6b).
Measurement of MDA
As-intoxicated rats displayed potential oxidative stress through a significant increase in MDA level (22.15 ± 0.35, P ≤ 0.001) compared to control animals (4.76 ± 0.96). Treatment of animals with SBE.CNPs demonstrated a significant decrease in MDA contents (10.10 ± 0.56, P ≤ 0.001) relative to As-intoxicated rats (Fig. 6c).
Effect on Apoptosis
As-intoxicated rats demonstrated a substantial increase in apoptotic activity (19.00 ± 2.82) compared to controls (3.50 ± 0.70). On the other hand, SBE-CNP administration can return the apoptotic activity in brain tissue to normal levels (6.00 ± 1.41) compared to As-intoxicated rats (Fig. 7).
Effect of arsenic (As) and Salix subserrata bark extract-chitosan nanoparticle (SBE.CNPs) administration on the apoptotic activity of rats. Data are expressed as mean ± SEM, one-way ANOVA followed by post hoc test Tuckey test for eight rats in each group. *Significant from the control group and.$significant from As group. P < 0.05
Histopathological Analysis
Histopathological examination of brain tissues is presented in Fig. 8. The control group revealed a normal histological structure of different brain regions, including the cerebral cortex, hippocampus, and cerebellum. Briefly, the cerebral cortex showed intact basophilic neurons and neuroglia. Additionally, the hippocampus is divided into three areas as follows: hippocampus proper, dentate gyrus, and subiculum. The hippocampus proper is classified according to the size and shape of neurons into four fields (CA1–CA4). Finally, the cerebellum exhibited a well-defined molecular layer, granule cell layer, and Purkinje cell layer.
Examination the protective effect of Salix subserrata bark extract-chitosan nanoparticle (SBE.CNPs) against arsenic (As)-induced neurotoxicity. a Brain tissue stained with H&E. Notable marks on the figure indicate as follow: dark-stained neuron (black arrow), curved arrow (neurophagia of degenerated neuron), red arrow (karyolysis), blue arrow (karyorrhexis), arrowhead (necrotic neuron), star (gliosis), $ (area avoid from neurons). b–d Histological damage score of the cerebral cortex, hippocampus, and cerebellum, respectively. Data are expressed as median ± SD by using Kruskal–Wallis test, followed by the Dunn’s Multiple Comparison Test (*P < 0.05; ***P < 0.001)
In As-intoxicated rats, the cerebral cortex showed focal gliosis, darkly stained neurons, and neurophagia of degenerated neurons. Likewise, several histological alterations were observed in the hippocampal area, specifically in CA1 and dentate gyrus. The thickness of CA1 reduced into one or two raw besides karyolysis and karyorrhexis were detected in the neurons. Additionally, the diameter of the dentate gyrus shrunken combined with apoptotic changes in neurons. Unpredictably, no histological damage was detected in CA2, CA3, and CA4. The cerebellum of As-intoxicated rats showed marked Purkinje cell necrosis.
Administration of SBE maintained the neuronal structure besides restoring the thickness of both CA1 and dentate gyrus. Only a few sporadic neurophagia of degenerated neurons were detected in the cerebral cortex.
Immunohistochemistry Analysis
The expression of both NF-ƘB and HO-1 was significantly increased in As-intoxicated rats compared to the control group (P < 0.001, P < 0.01, respectively). SBE was found to downregulate the expression of both NF-ƘP and HO-1. Unexpectedly, no significant difference was recorded in these groups compared to the control group (Fig. 9).
Examination the effect of Salix subserrata bark extract-chitosan nanoparticle (SBE.CNPs) on the expression of nuclear factor kappa B (NF-ƘB) and heme oxygenase-1 (HO-1). a Immunohistochemical staining of brain tissue with NF-ƘB and OH-1. b, c Assessment the percent of expression area of NF-ƘB and HO-1, respectively. Results are presented as mean ± SD using one-way analysis of variance (ANOVA), next Tukey’s comparison test is applied. (**P < 0.01; ***P < 0.001)
Discussion
Several reports have shown that polyphenolic compounds isolated and/or identified in different organs of Salix species exert various biological activities [10]. These activities are restricted due to the slow absorption of most of the isolated active compounds owing to their high molecular weights [54]. Nanotechnology is an emerging science that solves plant extracts’ solubility, stability, and absorbability problems and, consequently, increases their therapeutic activities. Therefore, this study set out with the aim of developing and characterizing SBE.CNPs followed by in vivo evaluation of its neuroprotective activity against As-induced neurotoxicity in rats. This study was supported by HPLC–PDA-ESI–MS/MS characterization of the chemical composition of S. subserrata stem bark (B), shoots (S), and leaves (L) extracts and in vitro antioxidant activity evaluation.
The chemical composition of S. subserrata stem bark, shoot, and leaf extracts revealed different patterns. Interestingly, about 102 secondary metabolites were identified in S. subserrata; six of them (catechin, (epi)catechin-(epi)catechin, gallocatechin, myricetin-3-O-β-d-glucoside, chrysoeriol-7-O-glucuronide, and phenyl alanine) were previously isolated [38], while the others were reported for the first time in the current study. Intriguingly, a series of procyanidins dominated the stem bark extract (51% of total identified compounds), where about 14 dimeric proanthocyanidins and 13 trimeric proanthocyanidins were identified based on characteristic λ max at 280 nm and molecular ion peak. This is in agreement with the previous finding, where the chemical profiling of Salix tetrasperma bark extract revealed the presence of catechin monomers, dimers, and trimers [55]. Furthermore, both bark and leaf extract of Salix caprea L. extracts showed the accumulation of phenolic acids and flavonoids mainly rutin [56]. However, the phenolic compounds that were previously detected in Salix alba (L.) leaf extract are regarded mainly as flavonoids [57], while the chemical profile of the leaf extract of S. subserrata in the current study exhibited the presence of three major classes of compounds such as flavonoids that do not contain rutin, phenolic acids derivatives, and salicinoids that constitute 39%, 35%, and 20% of total identified compounds, respectively. Finally, salicinoids prevailed in the S. subserrata shoots (17% of total identified compounds); this is inconsistent with the published data that confirmed the detection of phenolic acids with some flavonoids and salicylates in young shoots of different Salix species [58].
The in vitro antioxidant activity of different parts of S. subserrata was evident. The bark extract exhibited the highest activity (IC 50 = 16.8 µg/m) relative to shoots and leaves. These results agreed with Ishikado et al. (2013), who demonstrated the antioxidant activities of S. purpurea, S. daphnoides, and S. fragilis bark extracts against oxidative stress-induced damage in human umbilical vein endothelial cells. In our study, we fabricated chitosan polymer to fabricate a nanoparticle delivery vehicle. Chitosan is a semisynthetic material obtained by the deacetylation of chitin and is comprised of glucosamine (deacetylated monomer) and N-acetyl-glucosamine (acetylated monomer) monomers linked through β-,4 glycosidic bonds [59].
Chitosan nanoparticles are biodegradable, more stable, simple, less toxic, biocompatible, and easy to prepare. Also, chitosan polymer is approved by Generally Recognized as Safe by the United States Food and Drug Administration [US FDA] [60]. Oral administration of chitosan nanoparticles increases the bioavailability of encapsulation due to its mucoadhesion properties and transient opening of the tight junctions of the mucosal cell membrane. Also, the interaction between chitosan (positively charged) and mucin (negatively charged) leads to an increase in contact time between the formulation and the absorptive surface [61]. Moreover, chitosan has amino group with the pKa of ~ 6.5 and has better permeation-enhancing properties, promoting drug absorption at the proximal part of the GI tract, including the stomach and duodenum (M. Ways et al., 2018). The usage of chitosan nanoparticles to deliver plant extract was in accordance with a previous study by Ali et al. [62].
The prepared SBE.CNPs showed spherical nanoparticles with an average size of 193.4 ± 24.5 nm and a zeta potential of + 39.6 ± 0.4 mV. These values are in agreement with typically observed chitosan nanoparticles synthesized by the ionic gelation technique [63]. The small size of SBE.CNPs depended on the chitosan concentration (2 mg/ml) during the synthesis process and the mass ratio of chitosan to TPP (3:1) where a higher concentration of TPP may lead to stronger intramolecular interaction of chitosan and consequently the formation of the smaller size of particles [4, 5]. Additionally, the pH of the chitosan solution at 5 was expected to control the homogeneity and the size of nanoparticles [10, 11]. The high positive zeta potential value may be attributed to amino groups on the surface of chitosan particles, which reduced the possibility of particle aggregation, consequently proving the stability of NPs. Despite adding anionic TPP to chitosan during the fabrication procedures, the produced nanoparticles kept a positive charge. This may be due to the high molecular weight of chitosan [64, 65].
CNPs also demonstrated high SBE loading with 83.7 ± 4.3%. The high loading of SBE is influenced by the increase of chitosan amount and relatively large particle size, which make nanoparticles encapsulate a higher amount of SBE because particle surface and volume had been increased. Additionally, the ratio between chitosan and TPP during nanoparticle fabrication controls the encapsulation ability; higher TPP content increases the amount of chitosan incorporated with TPP [19]. SBE initially burst released at first few hours which may be attributed to the leaking of weakly cross-linked molecules that resulted from the adsorption of some of SBE on the surface of NPs [66, 67]. After the burst release period, the release pattern was in a gradual decrease rate that may be attributed to changes in the release mechanism from SBE diffusion through the polymer matrix to SBE exposure due to polymer erosion [12].
Our in vivo study demonstrated that As exposure results in neurobehavioral, biochemical, and pathological alteration. The neurobehavioral changes include a highly anxious state evidenced by the increase in the time spent in the dark chamber associated with a decrease in the time spent in the light chamber of the light–dark activity box. Light dark activity box is a widely used test to detect the anxious state of rodents [68], and this result was in accordance with a previous study by [69]. Alongside, As-exposure results in locomotor, exploratory, and memory impairments as visualized by different behavioral tests (open field test and Y-maze test). These results were in agreement with earlier studies [69, 70]. These studies discussed the neurobehavioral impairments caused by arsenic and the anxiolytic and anti-inflammatory role of different compounds, including gallic acid [69] and thymoquinone [70]. On the other hand, the administration of SBE.CNPs reversed all the aforementioned behavioral changes conveyed by a highly anxiolytic state, and increased locomotion, exploration, and cognitive abilities.
As-induced behavioral deficits were supported by deleterious changes in brain tissue, including degeneration, apoptosis, and necrosis of the neurons. Several studies have reported that As could pass through the blood–brain barrier [71, 72] and induce neurotoxicity through either generation of the ROS [73] or stimulation of the mitochondrial damage [74, 75] that consequently stimulate the necrosis or apoptosis of the cell [74, 75]. Oxidative stress has been reported as one of the key responsible parameters for brain damage and neurodegeneration by oxidative injury through the over-production of the lipid peroxidation marker MDA and the reduced activity of several antioxidants, including GSH-Px and SOD.
SOD is the first member of the antioxidant defense system via catalyzing the dismutation of the superoxide anion to oxygen and hydrogen peroxide (H2O2), which is then metabolized by GSH-Px [76]. Furthermore, MDA is widely recognized as the most important product in the peroxidation of membrane lipids, and thus, it serves as an indirect indicator of the extent of cell damage [77]. In the present work, As-intoxication caused a significant decrease in antioxidant enzyme activities of GSH-Px, and SOD, as well as an increase in the MDA level. Interestingly, the activities of antioxidant enzymes were effectively increased by the administration of SBE.CNPs, which also significantly reduced the production of MDA in the brain tissue and consequently downregulated the percentage of apoptosis, suggesting that SBE.CNP administration has the potential for the prevention of neurodegenerative diseases. These results were in accordance with the DPPH assay outcomes and showed the possible mechanistic antioxidant pathway of SBE.CNPs. Also, they were in the same line with a previous study by Dwivedi et al. [78].
Moreover, ROS production is exaggerated by NF-ƘB which produces pro-inflammatory cytokines and cytotoxic genes [7]. In the current study, As-intoxicated rats exhibited an intensive immunohistochemical expression of NF-ƘB. This result is in agreement with a previously reported study [79]. At the same time, the extreme production of ROS impaired the degradation of Nrf2, which is mediated by kelch-like epichlorohydrin-associated protein 1 (Keap1) [80], resulting in the accumulation of Nrf2 that upregulated HO-1 production [81, 82], as confirmed in our study. Heme oxygenase-1 (HO-1) is considered a stress-responsive enzyme with a primary antioxidant and anti-inflammatory role which is capable of catabolizing heme into iron, carbon monoxide, and biliverdin. Thus, it has an important role in brain protection. Several studies highlighted that induction of HO-1 is triggered by its substrate heme as well as by biological, chemical, and physiological stress conditions brought on by toxic concentrations of drugs or metals, and consequently, the relief of the stress condition can downregulate the HO-1 levels again [83]. This was in the same line with the current findings which revealed the upregulation of HO-1 in As-intoxicated rats.
On the other hand, the administration of SBE.CNPs can maintain the cellular structure of the neurons against As-induced neurotoxicity, in addition to suppressing the expression of both NF-ƘB and HO-1. These results could be attributed to the antioxidant effect of SBE.CNPs, besides the ability to regulate multiple pathways such as NF-ƘB, Nrf2, and HO-1. The antioxidant and anti-inflammatory activities of the genus Salix were previously reported, where S. subserrata, S. tetrasperma, and purified salicin exerted promising anti-inflammatory activity [10, 11, 38]. In addition, phenolic compounds (matsudone A, 4′,7-dihydroxyflavone, isoquercitrin, 7-methoxyflavone, and luteolin-7-O-glucoside) isolated from S. matsudana have anti-inflammatory activity [84, 85].
Moreover, it was previously reported that medicinal plants containing proanthocyanidins possessed powerful antioxidants, and anti-inflammatory, increase clinical health benefits and prevent age-related diseases. In addition, proanthocyanidins as polyphenolics showed neuroprotective effects against several neurotoxins (β-amyloid25-35, pentylenetetrazole, and rotenone) that caused negatively modulating apoptotic signaling pathways, alleviating oxidative and inflammatory damage [86,87,88,89,90,91,92,93]. Intriguingly, our study tested for the first time the effects of proanthocyanidin-bearing plants (S. subserrata) in As-induced neurotoxicity, and astonishing results were obtained. However, further studies will be recommended for determining the effect of co-administrating of SBE during other chelating therapies as a thiol chelator and the molecular mechanism underlying the potential neuroprotective effects.
Conclusion
In conclusion, chitosan, a simple, less-toxic, and biodegradable biopolymer, was used as a vehicle for the crude extract of S. subserrata bark (SBE.CNPs). The prepared SBE.CNPs were thoroughly characterized by SEM, DLS, PDI, and zeta potential techniques. The stem bark was selected for NP formulation based on HPLC–PDA-ESI–MS/MS profiling and its potent antioxidant ability using in vitro free radical scavenging activity. The study was extended to examine the neuroprotective activity of the prepared SBE.CNPs against As-induced neurotoxicity. Our obtained data revealed that the administration of SBE.CNPs counteract the As-induced neurotoxicity by reversing the behavioral deficits, including locomotion, exploration, and memory impairments. Also, it boosted the levels of SOD and GSH-Px and reduced the MDA levels associated with normalizing apoptosis in brain cells. Moreover, it maintained the brain’s architecture, accompanied by suppressing the expression of both NF-ƘB and HO-1. Therefore, chitosan could be used as a safer environmentally benign carrier for the SBE that could be used as a natural drug against As-induced neurotoxicity.
Data Availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Change history
29 June 2023
A Correction to this paper has been published: https://doi.org/10.1208/s12249-023-02508-9
References
Kuivenhoven M, Mason K. Arsenic Toxicity. StatPearls [Internet]. Treasure island (FL): StatPearls Publishing; 2021. Available from: http://www.ncbi.nlm.nih.gov/books/NBK541125/. Accessed 28 June 2022.
Wu X, Cobbinaauthor SJ, Maoauthor G, Xuauthor H, Zhangauthor Z, Yangauthor L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. 2015 [cited 2021 Nov 19]; Available from: https://agris.fao.org/agris-search/search.do?recordID=US201600233474. Accessed 28 June 2022.
Rao CV, Pal S, Mohammed A, Farooqui M, Doescher MP, Asch AS, et al. Biological effects and epidemiological consequences of arsenic exposure, and reagents that can ameliorate arsenic damage in vivo. Oncotarget Impact Journals. 2017;8:57605–21.
Tolins M, Ruchirawat M, Landrigan P. The developmental neurotoxicity of arsenic: cognitive and behavioral consequences of early life exposure. Ann Glob Health. 2014;80:303–14.
Yen CC, Ho TJ, Wu CC, Chang CF, Su CC, Chen YW, et al. Inorganic arsenic causes cell apoptosis in mouse cerebrum through an oxidative stress-regulated signaling pathway. Arch Toxicol. 2011;85:565–75.
Singh AP, Goel RK, Kaur T. Mechanisms pertaining to arsenic toxicity. Toxicol Int. 2011;18:87–93.
Hu Y, Li J, Lou B, Wu R, Wang G, Lu C, et al. The role of reactive oxygen species in arsenic toxicity. Biomolecules. 2020;10:240.
Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduct Target Ther. Nat Publ Group. 2017;2:1–9.
Hayden MS, Ghosh S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–34.
Tawfeek N, Mahmoud MF, Hamdan DI, Sobeh M, Farrag N, Wink M, et al. Phytochemistry, pharmacology and medicinal uses of plants of the genus salix: an updated review. Front Pharmacol [Internet]. 2021 [cited 2021 Dec 19];0. Available from: https://doi.org/10.3389/fphar.2021.593856/full
Kishore RN, Mangilal T, Anjaneyulu N, Abhinayani G, Sravya N. Investigation of anti-inflammatory and invitro antioxidant activities of hydroalcoholic extract of bark of Salix tetrasperma Roxb. Int J Pharm Drug Anal. 2014;2:506–9.
Popova TP, Kaleva MD. Antimicrobial effect in vitro of aqueous extracts of leaves and branches of willow (Salix babylonica L.). Int J Curr Microbiol Appl Sci. 2015;4:146–52.
Mohammed MA, Syeda JTM, Wasan KM, Wasan EK. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics. 2017;9:53.
Lopes VF, Giongo CN, de Almeida Campos L, Abraham W-R, Mainardes RM, Khalil NM. Chitosan nanoparticles potentiate the in vitro and in vivo effects of curcumin and other natural compounds [Internet]. 2021 [cited 2021 Dec 19]. Available from: https://www.ingentaconnect.com/content/ben/cmc/2021/00000028/00000024/art00008;jsessionid=8mcm5oto21fh6.x-ic-live-01. Accessed 28 June 2022.
Sharifi-Rad J, Quispe C, Butnariu M, Rotariu LS, Sytar O, Sestito S, et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021;21:318.
Bahram M, Mohammadzadeh E. Green synthesis of gold nanoparticles with willow tree bark extract: a sensitive colourimetric sensor for cysteine detection. Anal Methods. R Soc Chemi. 2014;6:6916–24.
Islam NU, Jalil K, Shahid M, Rauf A, Muhammad N, Khan A, et al. Green synthesis and biological activities of gold nanoparticles functionalized with Salix alba. Arab J Chem. 2019;12:2914–25.
Ghareeb MA, Mohamed T, Saad AM, Refahy LA-G, Sobeh M, Wink M. HPLC-DAD-ESI-MS/MS analysis of fruits from Firmiana simplex (L.) and evaluation of their antioxidant and antigenotoxic properties. J Pharm Pharmacol. 2017;70:133–42.
Nair RS, Morris A, Billa N, Leong C-O. An evaluation of curcumin-encapsulated chitosan nanoparticles for transdermal delivery. AAPS PharmSciTech. 2019;20:69.
Steven Leary WU, Raymond A, Cartner S, Corey D, Grandin T, Greenacre C, et al. AVMA Guidelines for the Euthanasia of Animals. Schaumburg, IL: American Veterinary Medical Association; 2013.
Tandon N, Roy M, Roy S, Gupta N. Protective effect of psidium guajava in arsenic-induced oxidative stress and cytological damage in rats. Toxicol Int. 2012;19:245–9.
Khalil HMA, Eliwa HA, El-Shiekh RA, Al-Mokaddem AK, Hassan M, Tawfek AM, et al. Ashwagandha (Withania somnifera) root extract attenuates hepatic and cognitive deficits in thioacetamide-induced rat model of hepatic encephalopathy via induction of Nrf2/HO-1 and mitigation of NF-kB/MAPK signaling pathways. J Ethnopharmacol. 2021;277:114141.
Khalil HMA, Salama HH, Al-Mokaddem AK, Aljuaydi SH, Edris AE. Edible dairy formula fortified with coconut oil for neuroprotection against aluminium chloride-induced Alzheimer’s disease in rats. J Funct Foods. 2020;75:104296.
Bancroft JD, Gamble M. Theory and practice of histology techniques. Elsevier Health Sciences; 2008. p. 83–134.
Falcão S, Vale N, Gomes P, Domingues M, Freire C, Sm S, et al. Phenolic profiling of Portuguese propolis by LC-MS spectrometry: uncommon propolis rich in flavonoid glycosides [Internet]. Phytochem. Anal. PCA. 2013 [cited 2020 May 26]. Available from: https://pubmed.ncbi.nlm.nih.gov/23172843/. Accessed 28 June 2022.
Sun J, Kou L, Geng P, Huang H, Yang T, Luo Y, et al. Metabolomic assessment reveals an elevated level of glucosinolate content in CaCl2 treated broccoli microgreens. J Agric Food Chem. 2015;63:1863–8.
Karar MGE, Pletzer D, Jaiswal R, Weingart H, Kuhnert N. Identification, characterization, isolation and activity against Escherichia coli of quince (Cydonia oblonga) fruit polyphenols. Food Res Int. 2014;65:121–9.
Jaiswal R, Jayasinghe L, Kuhnert N. Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC–MS. J Mass Spectrom. 2012;47:502–15.
Li S, Lin Z, Jiang H, Tong L, Wang H, Chen S. Rapid identification and assignation of the active ingredients in Fufang Banbianlian injection using HPLC-DAD-ESI-IT-TOF-MS. J Chromatogr Sci. 2016;54:1225–37.
Friedrich W, Eberhardt A, Galensa R. Investigation of proanthocyanidins by HPLC with electrospray ionization mass spectrometry. Eur Food Res Technol. 2000;211:56–64.
Piraud M, Vianey-Saban C, Petritis K, Elfakir C, Steghens J-P, Morla A, et al. ESI-MS/MS analysis of underivatised amino acids: a new tool for the diagnosis of inherited disorders of amino acid metabolism. Fragmentation study of 79 molecules of biological interest in positive and negative ionisation mode. Rapid Commun Mass Spectrom. 2003;17:1297–311.
Abu-Reidah IM, Ali-Shtayeh MS, Jamous RM, Arráez-Román D, Segura-Carretero A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015;166:179–91.
El-Wakil EA, Abdel-Hameed E-SS, El-Sayed MM, Abdel-Lateef EE. Identification of the chemical composition of the methanolic extract of Salix tetrasperma Roxb. using LC-ESI-MS and evaluation its potential as antioxidant agent. Der Pharma Chemica. 2015;7:168–167.
Parveen I, Threadgill MD, Hauck B, Donnison I, Winters A. Isolation, identification and quantitation of hydroxycinnamic acid conjugates, potential platform chemicals, in the leaves and stems of Miscanthus×giganteus using LC–ESI-MSn. Phytochemistry. 2011;72:2376–84.
Tala VRS, Candida da Silva V, Rodrigues CM, Nkengfack AE, Campaner dos Santos L, Vilegas W. Characterization of proanthocyanidins from Parkia biglobosa (Jacq.) G. Don.(Fabaceae) by flow injection analysis—electrospray ionization ion trap tandem mass spectrometry and liquid chromatography/electrospray ionization mass spectrometry. Molecules. 2013;18:2803–20.
Keefover-Ring K, Ahnlund M, Abreu IN, Jansson S, Moritz T, Albrectsen BR. No evidence of geographical structure of salicinoid chemotypes within Populus tremula. PLoS ONE. 2014;9: e107189.
Pearl IA, Darling SF. Purpurein, a new glucoside from the bark of Salix purpurea. Phytochemistry. 1970;9:853–6.
Tawfeek N, Sobeh M, Hamdan DI, Farrag N, Roxo M, El-Shazly AM, et al. Phenolic compounds from Populus alba L. and Salix subserrata Willd. (Salicaceae) counteract oxidative stress in Caenorhabditis elegans. Molecules. 2019;24:1999.
Zhang X, Lin Z, Fang J, Liu M, Niu Y, Chen S, et al. An on-line high-performance liquid chromatography–diode-array detector–electrospray ionization–ion-trap–time-of-flight–mass spectrometry–total antioxidant capacity detection system applying two antioxidant methods for activity evaluation of the edible flowers from Prunus mume. J Chromatogr A. 2015;1414:88–102.
Spínola V, Pinto J, Castilho PC. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD–ESI-MSn and screening for their antioxidant activity. Food Chem. 2015;173:14–30.
Chen G, Li X, Saleri F, Guo M. Analysis of flavonoids in Rhamnus davurica and its antiproliferative activities. Molecules. 2016;21:1275.
Alakolanga A, Siriwardane A, Kumar NS, Jayasinghe L, Jaiswal R, Kuhnert N. LC-MSn identification and characterization of the phenolic compounds from the fruits of Flacourtia indica (Burm. F.) Merr. and Flacourtia inermis Roxb. Food Res Int. 2014;62:388–96.
Kang J, Price WE, Ashton J, Tapsell LC, Johnson S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016;211:215–26.
Si C-L, Kim J-K, Bae Y-S, Li S-M. Phenolic compounds in the leaves of Populus ussuriensis and their antioxidant activities. Planta Med. 2009;75:1165–7.
Iwashina T, Peng C-I, Kokubugata G. Flavone O-and C-glycosides from Pothos chinensis (Araceae). Bull Natl Mus Nat Sci. 2010;36:27–32.
Boeckler GA, Gershenzon J, Unsicker SB. Phenolic glycosides of the Salicaceae and their role as anti-herbivore defenses. Phytochemistry. 2011;72:1497–509.
Jaiswal R, Matei MF, Subedi P, Kuhnert N. Does roasted coffee contain chlorogenic acid lactones or/and cinnamoylshikimate esters? Food Res Int. 2014;61:214–27.
Ağalar HG, Çiftçi GA, Göger F, Kırımer N. Activity guided fractionation of Arum italicum Miller Tubers and the LC/MS-MS profiles. Rec Nat Prod. 2017;12:64–75.
Kammerer B, Kahlich R, Biegert C, Gleiter CH, Heide L. HPLC-MS/MS analysis of willow bark extracts contained in pharmaceutical preparations. Phytochem Anal. 2005;16:470–8.
Dagvadorj E, Shaker KH, Windsor D, Schneider B, Boland W. Phenolic glucosides from Hasseltia floribunda. Phytochemistry. 2010;71:1900–7.
Yang N-Y, Yang Y-F, Li K. Analysis of hydroxy fatty acids from the pollen of Brassica campestris L. var. oleifera DC. by UPLC-MS/MS. J Pharm [Internet]. 2013;2013. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4595942/. Accessed 28 June 2022.
Dimkić I, Ristivojević P, Janakiev T, Berić T, Trifković J, Milojković-Opsenica D, et al. Phenolic profiles and antimicrobial activity of various plant resins as potential botanical sources of Serbian propolis. Ind Crops Prod. 2016;94:856–71.
Balbaa SI, Khafagy SM, Haggag MY, Sahsah NA. Phytochemical study of certain Salix species cultivated in Egypt. Egypt J Pharm Sci [Internet]. 1982 [cited 2021 Nov 12]; Available from: https://agris.fao.org/agris-search/search.do?recordID=US201302184926. Accessed 28 June 2022
Mamillapalli V. Nanoparticles for herbal extracts. Asian J Pharm AJP Free Full Text Artic Asian J Pharm [Internet]. 2016 [cited 2021 Nov 28];10. Available from: http://www.asiapharmaceutics.info/index.php/ajp/article/view/623. Accessed 28 June 2022
Mostafa I, Abbas HA, Ashour ML, Yasri A, El-Shazly AM, Wink M, et al. Polyphenols from Salix tetrasperma impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Molecules. Multidisci Digit Publ Inst. 2020;25:1341.
Gligorić E, Igić R, Suvajdžić L, Teofilović B, Turk-Sekulić M, Grujić-Letić N. Methodological aspects of extraction, phytochemical characterization and molecular docking studies of Salix caprea L. bark and leaves. Acta Chim Slov. 2019;66:821–30.
Piątczak E, Dybowska M, Płuciennik E, Kośla K, Kolniak-Ostek J, Kalinowska-Lis U. Identification and accumulation of phenolic compounds in the leaves and bark of Salix alba (L.) and their biological potential. Biomolecules. Multidiscipl Dig Publ Inst. 2020;10:1391.
Budny M, Zalewski K, Stolarski MJ, Wiczkowski W, Okorski A, Stryiński R. The phenolic compounds in the young shoots of selected willow cultivars as a determinant of the plants’ attractiveness to cervids (Cervidae, Mammalia). Biology. Multidiscip Digit Publ Inst. 2021;10:612.
Sudhakar YN, Selvakumar M, D. Krishna B. Biopolymer electrolytes: fundamentals and applications in energy storage. Elsevier; 2018.
Pangestuti R, Kim S-K. Neuroprotective properties of chitosan and its derivatives. Mar Drugs. 2010;8:2117–28.
Garg U, Chauhan S, Nagaich U, Jain N. Current advances in chitosan nanoparticles based drug delivery and targeting. Adv Pharm Bull. 2019;9:195–204.
Ali KA, El-Naa MM, Bakr AF, Mahmoud MY, Abdelgawad EM, Matoock MY. The dual gastro- and neuroprotective effects of curcumin loaded chitosan nanoparticles against cold restraint stress in rats. Biomed Pharmacother Biomedecine Pharmacother. 2022;148: 112778.
Bugnicourt L, Alcouffe P, Ladavière C. Elaboration of chitosan nanoparticles: favorable impact of a mild thermal treatment to obtain finely divided, spherical, and colloidally stable objects. Colloids Surf Physicochem Eng Asp. 2014;457:476–86.
Miladi K, Sfar S, Fessi H, Elaissari A. Enhancement of alendronate encapsulation in chitosan nanoparticles. J Drug Deliv Sci Technol. 2015;30:391–6.
Silva CA, Nobre TM, Pavinatto FJ, Oliveira ON. Interaction of chitosan and mucin in a biomembrane model environment. J Colloid Interface Sci. 2012;376:289–95.
Almutairi FM, El Rabey HA, Tayel AA, Alalawy AI, Al-Duais MA, Sakran MI, et al. Augmented anticancer activity of curcumin loaded fungal chitosan nanoparticles. Int J Biol Macromol. 2020;155:861–7.
Khan MdA, Zafaryab Md, Mehdi SH, Ahmad I, Rizvi MMA. Characterization and anti-proliferative activity of curcumin loaded chitosan nanoparticles in cervical cancer. Int J Biol Macromol. 2016;93:242–53.
Arrant AE, Schramm-Sapyta NL, Kuhn CM. Use of the light/dark test for anxiety in adult and adolescent male rats. Behav Brain Res. 2013;256:119–27.
Samad N, Jabeen S, Imran I, Zulfiqar I, Bilal K. Protective effect of gallic acid against arsenic-induced anxiety−/depression- like behaviors and memory impairment in male rats. Metab Brain Dis. 2019;34:1091–102.
Firdaus F, Zafeer MF, Ahmad M, Afzal M. Anxiolytic and anti-inflammatory role of thymoquinone in arsenic-induced hippocampal toxicity in Wistar rats. Heliyon. 2018;4: e00650.
Jin Y, Xi S, Li X, Lu C, Li G, Xu Y, et al. Arsenic speciation transported through the placenta from mother mice to their newborn pups. Environ Res. 2006;101:349–55.
Rodríguez VM, Carrizales L, Mendoza MS, Fajardo OR, Giordano M. Effects of sodium arsenite exposure on development and behavior in the rat. Neurotoxicol Teratol. 2002;24:743–50.
Demers-Lamarche J, Guillebaud G, Tlili M, Todkar K, Bélanger N, Grondin M, et al. Loss of mitochondrial function impairs lysosomes *. J Biol Chem. 2016;291:10263–76.
Flora SJS. Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med. 2011;51:257–81.
Parodi-Rullán RM, Soto-Prado J, Vega-Lugo J, Chapa-Dubocq X, Díaz-Cordero SI, Javadov S. Divergent effects of cyclophilin-D inhibition on the female rat heart: acute versus chronic post-myocardial infarction. Cell Physiol Biochem. 2018;50:288–303.
Zhen Y-Z, Lin Y-J, Li K-J, Zhang G-L, Zhao Y-F, Wang M-M, et al. Effects of rhein lysinate on D-galactose-induced aging mice. Exp Ther Med. 2016;11:303–8.
Senn A-C, Kaegi R, Hug SJ, Hering JG, Mangold S, Voegelin A. Effect of aging on the structure and phosphate retention of Fe(III)-precipitates formed by Fe(II) oxidation in water. Geochim Cosmochim Acta. 2017;202:341–60.
Dwivedi N, Mehta A, Yadav A, Binukumar BK, Gill KD, Flora SJS. MiADMSA reverses impaired mitochondrial energy metabolism and neuronal apoptotic cell death after arsenic exposure in rats. Toxicol Appl Pharmacol Academic Press. 2011;256:241–8.
Zhao H, Wang Y, Liu J, Guo M, Fei D, Yu H, et al. The cardiotoxicity of the common carp (Cyprinus carpio) exposed to environmentally relevant concentrations of arsenic and subsequently relieved by zinc supplementation. Environ Pollut. 2019;253:741–8.
Jiang J, Tam LM, Wang P, Wang Y. Arsenite targets the RING finger domain of Rbx1 E3 ubiquitin ligase to inhibit proteasome-mediated degradation of Nrf2. Chem Res Toxicol. 2018;31:380–7.
Aono J, Yanagawa T, Itoh K, Li B, Yoshida H, Kumagai Y, et al. Activation of Nrf2 and accumulation of ubiquitinated A170 by arsenic in osteoblasts. Biochem Biophys Res Commun. 2003;305:271–7.
Pi J, Diwan BA, Sun Y, Liu J, Qu W, He Y, et al. Arsenic-induced malignant transformation of human keratinocytes: involvement of Nrf2. Free Radic Biol Med. 2008;45:651–8.
Mackern-Oberti JP, Obreque J, Méndez GP, Llanos C, Kalergis AM. Carbon monoxide inhibits T cell activation in target organs during systemic lupus erythematosus. Clin Exp Immunol. 2015;182:1–13.
Bonaterra GA, Heinrich EU, Kelber O, Weiser D, Metz J, Kinscherf R. Anti-inflammatory effects of the willow bark extract STW 33-I (Proaktiv®) in LPS-activated human monocytes and differentiated macrophages. Phytomedicine. 2010;17:1106–13.
Li X, Liu Z, Zhang X, Wang L, Zheng Y, Yuan C, et al. Isolation and Characterization of Phenolic Compounds from the Leaves of Salix matsudana. Molecules. 2008;13:1530–7.
Jiang Y, Yang W, Gui S. Procyanidin B2 protects rats from paraquat-induced acute lung injury by inhibiting NLRP3 inflammasome activation. Immunobiology. 2018;223:555–61.
Ma J, Gao S-S, Yang H-J, Wang M, Cheng B-F, Feng Z-W, et al. Neuroprotective effects of proanthocyanidins, natural flavonoids derived from plants, on rotenone-induced oxidative stress and apoptotic cell death in human neuroblastoma SH-SY5Y cells. Front Neurosci [Internet]. 2018;12. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5990600/. Accessed 28 June 2022.
Jin W, Sun M, Yuan B, Wang R, Yan H, Qiao X. Neuroprotective effects of grape seed procyanidins on ethanol-induced injury and oxidative stress in rat hippocampal neurons. Alcohol Alcohol. 2020;55:357–66. Accessed 28 June 2022
Chen J, Chen Y, Zheng Y, Zhao J, Yu H, Zhu J, et al. Neuroprotective effects and mechanisms of procyanidins in vitro and in vivo. Molecules. 2021;26:2963.
Ishikado A, Sono Y, Matsumoto M, Robida-Stubbs S, Okuno A, Goto M, et al. Willow bark extract increases antioxidant enzymes and reduces oxidative stress through activation of Nrf2 in vascular endothelial cells and Caenorhabditis elegans. Free Radic Biol Med. 2013;65:1506–15.
Fahmy IR, Abdel‐Latif IA. A comparative study of Salix species grown in Egypt**Abstract of thesis offered in partial fulfillment of the requirements for the Degree of Master of Pharmacy, Fouad I University, February 1943, Cairo, Egypt.††Presented to the Scientific Section, A. PH. A., Milwaukee meeting, August, 1947. J Am Pharm Assoc Sci Ed. 1948;37:276–83.
Al-Sherif EA, Amer W, Khodary SEA, Azmy W. Ecological studies on Salix distribution in Egypt. Asian J Plant Sci ANSInet, Asian Netw Sci Inf. 2009;8:230–4.
M. Ways TM, Lau WM, Khutoryanskiy VV. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers. Multidiscip Digit Publ Inst. 2018;10:267.
Acknowledgements
Not applicable
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Daila Ibrahim Hamdan, Nora Tawfeek, Riham Adel Tawfik Hassan El-Shiekh, Heba Muhammed Ali Khalil, Nawaal Farrag, Michael Wink, and Assem Mohamed El-Shazly: conceptualization, methdology, writing—original draft, review and editing. Mohamed Yehia Mahmoud and Dalia Zaafar: biochemcial analyses, writing—original draft. Alaa Fouad Bakr: histopathological investigation, writing—original draft, review and editing. Heba Muhammed Ali Khalil: behavioral analysis, methdology, formal analysis. Mohamed Yehia Mahmoud: investigation, preparation, and characterization of nanoformulation.
Corresponding authors
Ethics declarations
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original article has been updated to correct Mohamed Y. Mahmoud’s affiliation.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamdan, D.I., Tawfeek, N., El-Shiekh, R.A. et al. Salix subserrata Bark Extract-Loaded Chitosan Nanoparticles Attenuate Neurotoxicity Induced by Sodium Arsenate in Rats in Relation with HPLC–PDA-ESI–MS/MS Profile. AAPS PharmSciTech 24, 15 (2023). https://doi.org/10.1208/s12249-022-02478-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02478-4