Abstract
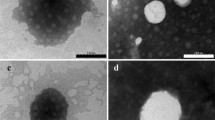
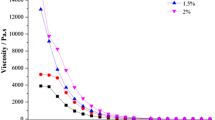
The purpose of this study was to design an in situ liquid crystal gel (ISLG) as an ophthalmic drug delivery system for dexamethasone (DEX) to enhance its eye retention and ocular bioavailability. The in situ liquid crystal gels (ISLGs) were prepared using a phytantriol/PEG400/water (65:30:5, w/w) ternary system. Polarized light microscope (PLM), small-angle X-ray scattering (SAXS), and rheology analysis confirmed that the internal structure of the preparations was Pn3m cubic phase liquid crystal gels with pseudoplastic fluid properties. Meanwhile, in vitro release behavior of the preparations conforms to the Higuchi equation. Corneal penetration experiments showed that compared with DEX sodium phosphate eye drops, DEX-ISLGs(F2) produced a 5.45-fold increase in the Papp value, indicating a significant enhancement of corneal penetration. In addition, in vivo experiments have confirmed that the ISLGs have better biocompatibility and longer retention time in the cornea. Simultaneously, corneal hydration level, eye irritation experiments, and histological observations proved the safety of the preparations. Pharmacokinetic studies have shown that the ISLG could maintain the DEX concentration in aqueous humor for at least 12 h after administration, which significantly improves the bioavailability of the drug. Collectively, these results indicated that ISLG would be a potential drug carrier for the treatment of diabetic retinopathy (DR).











Similar content being viewed by others
Availability of Data and Material
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Saaddine JB, Honeycutt AA, Narayan KM, Zhang X, Klein R, Boyle JP. Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005-2050. Arch Ophthalmol. 2008;126(12):1740–7.
Mahaling B, Srinivasarao DA, Raghu G, Kasam RK, Bhanuprakash Reddy G, Katti DS. A non-invasive nanoparticle mediated delivery of triamcinolone acetonide ameliorates diabetic retinopathy in rats. Nanoscale. 2018;10(35):16485–98.
Oussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18(12):1450–2.
Oussen AM, Poulaki V, Qin W, et al. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol. 2002;160(2):501–9.
Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2(14):e93751.
Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–203.
Jiang Y. Effect of total retinal laser photocoagulation on diabetic retinopathy. Med Equip. 2020;21:1002anibiz.
Bi SS, Ping JH, Dai X. Progress in the treatment of diabetic retinopathy with retinal laser photocoagulation. J Heze Med Coll. 2020;123:72–5.
Kleinmann G, Hauser D, Schechtman E, Landa G, Bukelman A, Pollack A. Vitreous hemorrhage in diabetic eyes previously treated with panretinal photocoagulation. Int Ophthalmol. 2008;28(1):29–34.
Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103(12):1796-1806.
Brănişteanu DC, Bilha A, Moraru A. Vitrectomy surgery of diabetic retinopathy complications. Rom J Ophthalmol. 2016;60(1):31–6.
Veritti D, Sarao V, Lanzetta P. A propensity-score matching comparison between 27-gauge and 25-gauge vitrectomy systems for the repair of primary rhegmatogenous retinal detachment. J Ophthalmol. 2019;2019:3120960 Published 2019 Jan 10.
Naruse Z, Shimada H, Mori R. Surgical outcomes of 27-gauge and 25-gauge vitrectomy day surgery for proliferative diabetic retinopathy. Int Ophthalmol. 2019;39(9):1973–80.
Tuuminen R, Sahanne S, Haukka J, Loukovaara S. Improved outcome after primary vitrectomy in diabetic patients treated with statins. Eur J Ophthalmol. 2016;26(2):174–81.
Yamada Y, Suzuma K, Ryu M, Tsuiki E, Fujikawa A, Kitaoka T. Systemic factors influence the prognosis of diabetic macular edema after pars plana vitrectomy with internal limiting membrane peeling. Curr Eye Res. 2013;38(12):1261–5.
Lv J, Chen MM, Mu ZH, Wang F, Qian ZY, Zhou L, Guo QT, Zhao ZM, Pan YP, Liao XY, Yang ZH, Cai N, Li SD, Zou YY. Intravitreal bevacizumab injection attenuates diabetic retinopathy in adult rats with experimentally induced diabetes in the early stage. J Diabetes Res. 2018;2018:9216791–18.
Striglia E, Caccioppo A, Castellino N, Reibaldi M, Porta M. Emerging drugs for the treatment of diabetic retinopathy. Expert Opin Emerg Drugs. 2020;25(3):261–71.
Liu L, Tham YC, Cheng CY. Intravitreal aflibercept for proliferative diabetic retinopathy. Lancet. 2017;390(10108):2140–1.
Yilmaz T, Cordero-Coma M, Lavaque AJ, Gallagher MJ, Padula WV. Triamcinolone and intraocular sustained-release delivery systems in diabetic retinopathy. Curr Pharm Biotechnol. 2011;12(3):337–46.
Tost F, Kempin R, Grossjohann R, Herfurth S. Diabetische netzhauterkrankungen. Aktuelle aspekte in der therapie [diabetic retinopathy--current aspects of therapy]. Med Monatsschr Pharm. 2016;39(4):148–58.
Lakhani P, Patil A, Majumdar S. Recent advances in topical nano drug-delivery systems for the anterior ocular segment. Ther Deliv. 2018;9(2):137–53.
Phillips K, Katz HR. A comparison of the efficacy of dexamethasone and loteprednol on endotoxin-induced uveitis in rodents following topical ocular administration. Invest Ophthalmol Vis Sci. 2005;46:983.
Gan L, Han S, Shen J, Zhu J, Zhu C, Zhang X, Gan Y. Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: Improving preocular retention and ocular bioavailability. Int J Pharm. 2010;396(1-2):179–87.
Shah SS, Denham LV, Elison JR, Bhattacharjee PS, Clement C, Huq T, Hill JM. Drug delivery to the posterior segment of the eye for pharmacologic therapy. Expert Rev Ophthalmol. 2010;5(1):75–93.
Loftsson T, Stefánsson E. Cyclodextrins and topical drug delivery to the anterior and posterior segments of the eye. Int J Pharm. 2017;531(2):413–23.
Inokuchi Y, Hironaka K, Fujisawa T, Tozuka Y, Tsuruma K, Shimazawa M, Takeuchi H, Hara H. Physicochemical properties affecting retinal drug/coumarin-6 delivery from nanocarrier systems via eyedrop administration. Invest Ophthalmol Vis Sci. 2010;51(6):3162–70.
Mahaling B, Katti DS. Physicochemical properties of core-shell type nanoparticles govern their spatiotemporal biodistribution in the eye. Nanomedicine. 2016;12(7):2149–60.
Yang Z, Liang X, Jiang X, Guo J, Tao Y, Wang S, Cao Y, Gui S. Development and evaluation of minocycline hydrochloride-loaded in situ cubic liquid crystal for intra-periodontal pocket administration. Molecules. 2018;23(9):2275.
Chen Y, Ma P, Gui S. Cubic and hexagonal liquid crystals as drug delivery systems. Biomed Res Int. 2014;2014:815981–12.
Cao J, Huang J, Gui S, Chu X. Preparation, synergism, and biocompatibility of in situ liquid crystals loaded with sinomenine and 5-fluorouracil for treatment of liver cancer. Int J Nanomedicine. 2021;16:3725–39.
Shan QQ, Jiang XJ, Wang FY, Shu ZX, Gui SY. Cubic and hexagonal liquid crystals as drug carriers for the transdermal delivery of triptolide. Drug Deliv. 2019;26(1):490–8.
Ahmed TA, Aljaeid BM. A potential in situ gel formulation loaded with novel fabricated poly(lactide-co-glycolide) nanoparticles for enhancing and sustaining the ophthalmic delivery of ketoconazole. Int J Nanomedicine. 2017;12:1863–75.
Li Q, Cao J, Li Z, Chu X. Cubic liquid crystalline gels based on glycerol monooleate for intra-articular injection. AAPS PharmSciTech. 2018;19(2):858–65.
Chen Y, Liang X, Ma P, Tao Y, Wu X, Wu X, Chu X, Gui S. Phytantriol-based in situ liquid crystals with long-term release for intra-articular administration. AAPS PharmSciTech. 2015;16(4):846–54.
Shelley H, Rodriguez-Galarza RM, Duran SH, Abarca EM, Babu RJ. In situ gel formulation for enhanced ocular delivery of nepafenac. J Pharm Sci. 2018;107(12):3089–97.
Yu S, Wang QM, Wang X, Liu D, Zhang W, Ye T, Yang X, Pan W. Liposome incorporated ion sensitive in situ gels for opthalmic delivery of timolol maleate. Int J Pharm. 2015;480(1-2):128–36.
Allam A, Elsabahy M, El Badry M, Eleraky NE. Betaxolol-loaded niosomes integrated within pH-sensitive in situ forming gel for management of glaucoma. Int J Pharm. 2021;598:120380.
Lin S, Ge C, Wang D, Xie Q, Wu B, Wang J, Nan K, Zheng Q, Chen W. Overcoming the anatomical and physiological barriers in topical eye surface medication using a peptide-decorated polymeric micelle. ACS Appl Mater Interfaces. 2019;11(43):39603–12.
Chu X, Li Q, Gui S, Li Z, Cao J, Jiang J. Characterization and in vitro permeation study of cubic liquid crystal containing sinomenine hydrochloride. AAPS PharmSciTech. 2018;19(5):2237–46.
Jie H, Liu L, Shuangying G, Xingqi W, Rongfeng H, Yong Z, Chunling T, Mengqiu X, Xiaoqin C. A novel phytantriol-based in situ liquid crystal gel for vaginal delivery. AAPS PharmSciTech. 2019;20(5):185.
Wang X, Zhang Y, Huang J, Xia M, Liu L, Tian C, Hu R, Gui S, Chu X. Self-assembled hexagonal liquid crystalline gels as novel ocular formulation with enhanced topical delivery of pilocarpine nitrate. Int J Pharm. 2019;562:31–41.
Barauskas J, Landh T. Phase behavior of the phytantriol/water system. Langmuir .2013; 19:9562.
Mei L, Xie Y, Huang Y, Wang B, Chen J, Quan G, Pan X, Liu H, Wang L, Liu X, Wu C. Injectable in situ forming gel based on lyotropic liquid crystal for persistent postoperative analgesia. Acta Biomater. 2018;67:99–110.
Chu X, Wang X, Tian C, Liu L, Xia M, Jiang J, Gui S. Dual drug-loaded cubic liquid crystal gels for transdermal delivery: Inner structure and percutaneous mechanism evaluations. Drug Dev Ind Pharm. 2019;45(12):1879–88.
Chu XQ, Zhang Y, Huang J, Li Q, Li ZG, Jiang JQ, Gui SY. The effect of prescription on the framework of lipid matrix and in vitro properties. Curr Drug Deliv. 2019;16(8):737–50.
Bitan-Cherbakovsky L, Yuli-Amar I, Aserin A, Garti N. Solubilization of vitamin E into H(II) LLC mesophase in the presence and in the absence of vitamin C. Langmuir. 2010;26(5):3648–53.
Patere S, Newman B, Wang Y, Choi S, Vora S, Ma AWK, Jay M, Lu X. Influence of manufacturing process variables on the properties of ophthalmic ointments of tobramycin. Pharm Res. 2018;35(9):179.
Mahrhauser DS, Reznicek G, Kotisch H, Brandstetter M, Nagelreiter C, Kwizda K, Valenta C. Semi-solid fluorinated-DPPC liposomes: morphological, rheological and thermic properties as well as examination of the influence of a model drug on their skin permeation. Int J Pharm. 2015;486(1-2):350–5.
Fernández-Ferreiro A, González Barcia M, Gil-Martínez M, Vieites-Prado A, Lema I, Argibay B, Blanco Méndez J, Lamas MJ, Otero-Espinar FJ. In vitro and in vivo ocular safety and eye surface permanence determination by direct and magnetic resonance imaging of ion-sensitive hydrogels based on gellan gum and kappa-carrageenan. Eur J Pharm Biopharm. 2015;94:342–51.
Mannermaa E, Vellonen KS, Urtti A. Drug transport in corneal epithelium and blood-retina barrier: emerging role of transporters in ocular pharmacokinetics. Adv Drug Deliv Rev. 2006;58(11):1136–63.
Suhonen P, Järvinen T, Koivisto S, Urtti A. Different effects of pH on the permeation of pilocarpine and pilocarpine prodrugs across the isolated rabbit cornea. Eur J Pharm Sci. 1998;6(3):169–76.
Wu Y, Yao J, Zhou J, Dahmani FZ. Enhanced and sustained topical ocular delivery of cyclosporine a in thermosensitive hyaluronic acid-based in situ forming microgels. Int J Nanomedicine. 2013;8:3587–601.
Sun J, Zhou Z. A novel ocular delivery of brinzolamide based on gellan gum: In vitro and in vivo evaluation. Drug Des Devel Ther. 2018;12:383–9.
de la Fuente M, Raviña M, Paolicelli P, Sanchez A, Seijo B, Alonso MJ. Chitosan-based nanostructures: A delivery platform for ocular therapeutics. Adv Drug Deliv Rev. 2010;62(1):100–17.
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent the official views of Anhui University of Chinese Medicine.
Funding
The authors gratefully acknowledge support from the National Natural Science Foundation of China (No.81803831)
Author information
Authors and Affiliations
Contributions
Wenqing Wu: She has a huge contribution to the design and writing of the experiment.
Wenxuan Cao and Jingbao Chen: collected and processed the experimental data.
Ye Cai and Baoqi Dong: final approval of the version to be published.
Xiaoqin Chu: contributed experimental design and writing and a lot of opinions.
Corresponding author
Ethics declarations
Ethics Approval
Animals (certificate: AHUCM-rabbits-2020029) were purchased from Anhui Medical University Center (Hefei, China). All the experimental protocols were approved by the Committee on Ethics of Animal Experiments, Anhui University of Chinese Medicine (Hefei, China), and were conducted in compliance with the Guidelines for Animal Experiments of Anhui University of Chinese Medicine.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, W., Cao, W., Chen, J. et al. In Situ Liquid Crystal Gel as a Promising Strategy for Improving Ocular Administration of Dexamethasone: Preparation, Characterization, and Evaluation. AAPS PharmSciTech 23, 36 (2022). https://doi.org/10.1208/s12249-021-02193-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02193-6