Abstract
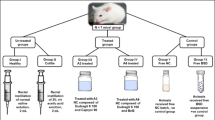
Oleogel consists of hydrophobic solvent and an oleogelator. In this study, attempts were made to study the influence of Celecoxib solubility, concentration and dispersability on its release, absorption, and biological performance. Oleogels were prepared to study the formulation variables on its stability and release. Castor oil was selected as the oil and the oleogelator concentration was 4.5% w/w. F3 revealed the highest release and stability compared to other formulae. The percent permeated across the rat intestine showed a 7.5-fold increase over free Celecoxib, and its lifetime was found to be greater than 18 months. The efficacy of free Celecoxib and oleogel formulae to treat rats with ulcerative colitis was done via the induction of ulcerative colitis (UC) through administration of 5% dextran sodium sulphate (DSS). Celecoxib besides its formulae significantly reduced the release of Leucine rich 2 glycoprotein (LRG), Myeloperoxidase (MPO), Tumor necrosis factor–α (TNF-α), proinflammatory cytokine expression, High mobility group box 1 (HMGB1), Nuclear factor kappa B (NF-ΚB), Trefoil Factor 3 (TFF3), Metalloproteinase-3 (MMP3), and miRNA31. Moreover, F3 significantly increased the colonic cAMP in DSS treated rats and reduced the intestinal inflammation beside healing of mucosa and restitution of the epithelium of the gastrointestinal tract.
Graphical abstract











Similar content being viewed by others
References
Patrignani P, Tacconelli S, Sciulli MG, Capone ML. New insights into COX-2 biology and inhibition. Brain Res Rev. 2005;48:352–9.
Graham D, Campen D, Hui R, Spence M, Cheetham C, Levy G, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and nonselective non-steroidal anti-inflammatory drugs: nested control study. Lancet. 2005;365:475–81.
O'Sullivan CM, Barbut S, Marangoni AG. Edible oleogels for the oral delivery of lipid soluble molecules: composition and structural design considerations. Trends Food Sci Technol. 2016;57:59–73.
Mohanty B, Pal K, Quereshi D, Nayak SK, Rathnam VSS, Banerjee I, et al. Oleogels based on palmitic acid and safflower oil: novel formulations for ocular drug delivery of voriconazole. Eur J Lipid Sci Technol. 2020;122:1–15.
Vintiloiu A, Leroux JC. Organogels and their use in drug delivery, a review. J Control Release. 2008;1253:179–92.
Murdan S. Organogels in drug delivery. Exp Opin Drug Deliv. 2005;2:1–17.
Balasubramanian R, Sughir AA, Damodar G. Oleogel: a promising base for transdermal formulations. Asian J Pharm. 2012;6:1–9.
Almeida IF, Bahia MF. Evaluation of the physical stability of two oleogels. Int J Pharm. 2006;327:73–7.
Goupale DC, Rajkapoor B. Improved stability and efficacy of diclofenac diethylamine in an oleogel based formulation. Ethiop Pharm J. 2008;26:125–8.
Badawi AA, Abd El-Aziz N, Amin MM, Sheta NM. Almond oil based oleogels as cosmetic bases for liposoluble sun screening agent bemotrizinol: in-vitro characterization and photostability. Inventi Rapid: Cosmeceuticals. 2015;2015(4):1–11.
Vintiloiu A, Lafleur M, Bastiat G, Leroux JC. In situ-forming oleogel implant for rivastigmine delivery. Pharm Res. 2008;25:845–52.
Feuerstein JD, Cheifetz AS. Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin Proc. 2014;89(11):1553–63.
Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014;7:113–20.
Park SC, Jeen YT. Genetic studies of inflammatory bowel disease-focusing on Asian patients. Cells. 2019;8(5):404.
Kangro HO, Chong SK, Hardiman A, Heath RB, Walker-Smith JA. A prospective study of viral and mycoplasma infections in chronic inflammatory bowel disease. Gastroenterology. 1990;98(3):549–53.
Khan RR, Lawson AD, Minnich LL, Martin K, Nasir A, Emmett MK, et al. Gastrointestinal norovirus infection associated with exacerbation of inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2009;48(3):328–33.
Lee SH, Kwon JE, Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018;16(1):26–42.
Balestrieri P, Ribolsi M, Pier M, Guarino L, Emerenziani S, Altomare A, et al. Nutritional aspects in inflammatory bowel diseases. Nutrients. 2020;12:372.
Abegunde AT, Muhammad BH, Bhatti OT. Environmental risk factors for inflammatory bowel diseases: evidence based literature review. World J Gastroenterol. 2016;22(27):6296–631.
Schmidt C, Giese T, Ludwig B, Molaian IM, Marth T, Zeuzem S, et al. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn's disease but not in ulcerative colitis. Inflamm Bowel Dis. 2005;11(1):16–23.
Westall FC. Integrating theories of the etiology of Crohn’s disease on the etiology of Crohn's disease: questioning the hypotheses. William M. Chamberlin, Saleh A. Naser Med Sci Monit 2006; 12 (2): RA27-33. Med SciMonit. 2006;12(5):LE5–6.
Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran Sulfate Sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:15–25.
Pinho RA, Silveira PC, Silva LA, Luiz Streck E, Dal-Pizzol F. N-acetylcysteine and deferoxamine reduce pulmonary oxidative stress and inflammation in rats after coal dust exposure. Environ Res. 2005;99:355–60.
Axelsson LG, Landstrom E, Goldschmidt TJ, Gronberg A, Bylund-Fellenius AC. Dextran sulfate sodium (DSS) induced experimental colitis in immunodeficient mice: effects in CD4(1)-cell depleted, athymic and NK-cell depleted SCID mice. Inflamm Res. 1996;45:181–91.
Miyazawa F, Olijnyk OR, Tilley CJ, Tamaoki T. Interactions between dextran sulfate and Escherichia coli ribosomes. Biochim Biophys Acta. 1967;145:96–104.
Kim JJ, Shajib MS, Manocha MM, Khan WI. Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp. 2012;60:e3678.
Cuzzocrea S, Mazzon E, Serraino I, Dugo L, Centorrino T, Ciccolo A, et al. A Celecoxib, a selective cyclo-oxygenase-2 inhibitor reduces the severity of experimental colitis induced by dinitrobenzene sulfonic acid in rats. Eur J Pharmacol. 2001;431:91–7.
Ghorab DM, Amin MM, Khowessah OM, Tadros MI. Colon targeted celecoxib-loaded Eudragit S100-coated poly-caprolactone microparticles: preparation, characterization and in vivo evaluation in rats. Drug Deliv. 2011;18:523–35.
Yang Y, Wang S, Xu H, Chengbo S, Xuanbin L, Zheng J. Properties of topically applied organogels: rheology and in vitro drug release. Asian J Pharm Sci. 2008;3:175–83.
Rushikesh P, Abraham S, Bharath S, Madhavan V. Sorbitan monostearate based organogels for topical delivery of clotrimazole. Int J Pharm Chem Sci. 2013;2:1246–52.
Sánchez R, Franco JM, Delgado MA, Valencia C, Gallegos C. Rheology of oleogels based on sorbitan and glyceryl monostearates and vegetable oils for lubricating applications. Grasas Aceites. 2011;62:328–36.
Sonar S, Gondkar S, Saudagar RB. Liquid filled hard gelatin capsule. J Drug Deliv Ther. 2019;9:832–5.
Ammar H, Ghorab M, Mostafa DM, Ghoneim AM. l. Self nanoemulsifying drug delivery system for sertraline hydrochloride: design, preparation and characterization. Int J Pharm. Pharm Sci. 2014;6(9):589–95.
El-Haddad AE, Sheta NM, Boshra SA. Isolation, formulation, and efficacy enhancement of morin emulsified carriers against lung toxicity in rats. AAPS PharmSciTech. 2018;19(5):2346–57.
Sheta NM, Elfeky YA, Boshra SA. Cardioprotective efficacy of silymarin liquisolid in isoproterenol prompted myocardial infarction in rats. AAPS PharmSciTech. 2020;21(81):1–16.
Kathpalia H, Sharma K, Doshi G. Recent trends in Hard Gelatin capsule delivery System. J Adv Pharm Edu Res. 2014;4(2):165–77.
Sahoo CK, Satyanarayana K, Bomma NG, Modugu KR, Nayak PK, Sarangi DK, et al. Formulation and evaluation of bifonazole organogel for the application of topical drug delivery system. Der Pharm Sin. 2013;4:67–74.
Sagiri SS, Behera B, Sudheep T, Pal K. Effect of composition on the properties of Tween-80–Span-80-based organogels. Des Mono Poly. 2012;15:253–73.
Kamble SR, Udapurkar P, Nakhat PD, Yeole PG, Biyani KR. Development and evaluation of sorbitan monostearate organogels as a topical delivery system for aceclofenac. Indian J Pharm Educ Res. 2011;45:65–70.
Chandran S, Ravi P, Saha RN. Development and in vitro evaluation of oral controlled release formulations of celecoxib using optimization techniques. YAKUGAKU ZASSHI (The Pharmaceutical Society of Japan). 2006;126(7):505–14.
Wang L, Dong J, Chen J, Eastoe J, Li X. Design and optimization of a new self-nanoemulsifying drug delivery system. J Colloid Interface Sci. 2009;330:443–8.
Madhusudhan B, Rambhau D, Apte SS, Gopinath D. Improved in vitro permeation of Nabumetone across rat skin from 1-O-/lecithin stabilized o/w nanoemulsions. J Dispers Sci Technol. 2006;27:921–6.
Ibrahim MM, Hafez SA, Mahdy MM. Organogels, hydrogels and bigels as transdermal delivery systems for diltiazem hydrochloride. Asian J Pharm Sci. 2013;8:48–57.
Prakash PR, Rao NGR, Soujanya C. Formulation, evaluation and anti-inflammatory activity of topical etoricoxib gel. Asian J Pharm Clin Res. 2010;3:126–9.
Tan SW, Billa N, Roberts CR, Burley JC. Surfactant effects on the physical characteristics of Amphotericin B-containing nanostructured lipid carriers. Coll Surf A: Physicochem Eng Aspects. 2010;372:73–9.
Mekkawy A, Fathy M, El-Shanawany S. Formulation and in vitro evaluation of fluconazole topical gels. Bri J Pharm Res. 2013;3:293–313.
Jibry N, Heenan RK, Murdan S. Amphiphilogels for drug delivery: formulation and characterization. Pharm Res. 2004;21:1852–61.
Benoit C, Jesse D, Madhu M, Matam V. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;25:1–15.
José W, Décio S, Mirian Z, Maria A, Rubens C. Comparison of selective and non-selective cyclo-oxygenase 2 inhibitors in experimental colitis exacerbation: role of leukotriene B4 and superoxide dismutase. Arq Gastroenterol. 2014;51:226–34.
Serada S, Fujimoto M, Ogata A, Terabe F, Hirano T, Iijima H. iTRAQ-based proteomic identification of leucine-rich alpha-2 glycoprotein as a novel inflammatory biomarker in autoimmune diseases. Ann Rheum Dis. 2010;69(4):770–4.
Kawakami M, Kaneko N, Anada H. Measurement of interleukin-6, interleukin-10, and tumor necrosis factor-alpha levels in tissues and plasma after thermal injury in mice. Surgery. 1997;121:440–8.
El-Ashmawy N, Khedr N, El-Bahrawy H, El-Adawy S. Type 4 phosphodiesterase inhibitor, attenuates inflammation in rats with ulcerative colitis via down-regulation of iNOS and elevation of Camp. Int Immunopharmacol. 2018;56:36–42.
Krawisz J, Sharon P, Stenson W. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity: assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–50.
Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75.
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179.
Malik MZ, Ahmad M, Minhas MU, Munir A. Solubility and permeability studies of aceclofenac in different oils. Trop J Pharm Res. 2014;13(3):327–30.
Barroso NG, Okuro PK, Ribeiro APB, Cunha RL. Tailoring properties of mixed-component oleogels: wax and monoglyceride interactions towards flaxseed oil structuring. Gels. 2020;6(5):1–20.
Ferro AC, Okuro PK, Badan AP, Cunha RL. Role of the oil on glyceryl monostearate based oleogels. Food Res Int. 2019;120:610–9.
Pènzes T, Blazsó G, Aigner Z, Falkay G, Erős I. Topical absorption of piroxicam from organogels—in vitro and in vivo correlations. Int J Pharm. 2005;298:47–54.
Huri MFD, Ng SF, Zulfakar MH. Fish oil-based oleogels: physicochemicals characterisation and in-vitro release of betamethasone dipropionate. Int J Pharm Pharm Sci. 2013;5:458–67.
Bhattacharya C, Kumar N, Sagiri SS, Pal K, Ray SS. Development of span 80–tween 80 based fluid-filled organogels as a matrix for drug delivery. J Pharm Bioallied Sci. 2012;4:155–63.
O'Laughlin R, Sachs C, Brittain H, Cohen E, Timmins P, Varia S. Effects of variations in physicochemical properties of glycerylmonostearate on the stability of an oil-in water cream. J Soc Cosmet Chem. 1989;40:215–29.
Jha S, Maurya SD. Organogels in drug delivery. J Biomed Pharm Res. 2013;2:89–99.
Wissing SA, Müller RH. Solid lipid nanoparticles as carrier for sunscreens: in-vitro release and in-vivo skin penetration. J Control Release. 2002;81:225–33.
Kogan A, Kesselman E, Danino D, Aserin A, Garti N. Viability and permeation across Caco-2 cells of CBZ solubilized in fully dilutable microemulsions. Colloids Surf B: Biointerfaces. 2008;66:1–12.
Kim MS, Jin SJ, Kim JS, Park HJ, Song HS, Neubert RHH, et al. Preparation, characterization and in vivo evaluation of amorphous atorvastatin calcium nanoparticles using supercrtitical antisolvent (SAS process). Eur J Pharm Biopharm. 2008;69:454–65.
Karade PG, Shah RR, Chougule DD, Bhise SB. Formulation and evaluation of celecoxib gel. J Drug Deliv Ther. 2012;2:132–5.
Yi EJ, Kim JY, Rhee YS, Kim SH, Lee HJ, Park CW, et al. Preparation of sildenafil citrate microcapsules and in-vitro/in-vivo evaluation of taste masking efficiency. Int J Pharm. 2014;466:286–95.
Liu Y, Sun C, Hao Y, Jiang T, Zheng L, Wang S. Mechanism of dissolution enhancement and bioavailability of poorly water soluble celecoxib by preparing stable amorphous nanoparticles. J Pharm Pharm Sci. 2010;13(4):589–606.
Hussain T, Saeed T, Mumtaz AM, Javaid Z, Abbas K, Awais A, et al. Effect of two hydrophobic polymers on the release of gliclazide from their matrix tablets. Acta Pol Pharm Drug Res. 2013;70:749–57.
Kanade R, Boche M, Pokharkar V. Self-assembling raloxifene loaded mixed micelles: formulation optimization, in vitro cytotoxicity and in vivo pharmacokinetics. AAPS PharmSciTech. 2018;19:1105–15.
Soliman SM, Sheta NM, Ibrahim BMM, El-Shawwa MM, Abd El-Halim SM. Novel intranasal drug delivery: geraniol charged polymeric mixed micelles for targeting cerebral insult as a result of ischaemia/reperfusion. Pharm. 2020;12(76):1–22.
Patel VR, Dumancas GG, Kasi Viswanath LC, Maples R, Subong BJ. Castor oil: properties, uses, and optimization of processing parameters in commercial production. Lipid insights. 2016;9:1–12.
Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–21.
Buchheister S, Buettner M, Basic M. CD14 plays a protective role in experimental inflammatory bowel disease by enhancing intestinal barrier function. Amer J Path. 2017;187:1106–20.
Cuzzocrea S, Mazzon E, Sautebin L, Dugo L, Serraino I, De Sarro A, et al. Protective effects of celecoxib on lung injury and red blood cells modification induced by carrageenan in the rat. Biochem Pharmacol. 2002;63:785–95.
Chan FK, Wong VW, Suen BY, Wu JC, Ching JY, Hung LC, et al. Combination of a cyclo oxygenase-2 inhibitor and a proton-pump inhibitor for prevention of recurrent ulcer bleeding in patients at very high risk: a double-blind, randomized trial. Lancet. 2007;369:1621–6.
Grønbaek H, Vestergaard EM, Hey H, Nielsen JN, Nexø E. Serum trefoil factors in patients with inflammatory bowel disease. Digest. 2006;74(1):33–9.
Palone F, Vitali R, Cucchiara S, Pierdomenico M, Negroni A, Aloi M, et al. Role of HMGB1 as a suitable biomarker of subclinical intestinal inflammation and mucosal healing in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2014;20(8):1448–57.
Tsutsumi R, Ito H, Hiramitsu T, Nishitani K, Akiyoshi M, Kitaori T, et al. Celecoxib inhibits production of MMP and NO via downregulation of NF-kappaB and JNK in a PGE2 independent manner in human articular chondrocytes. Rheumatol Int. 2008;28:727–36.
Alvarez-Soria MA, Herrero-Beaumont G, Moreno-Rubio J, Calvo E, Santillana J, Egido J, et al. Long-term NSAID treatment directly decreases COX-2 and mPGES-1 production in the articular cartilage of patients with osteoarthritis. Osteoarthr Cartil. 2008;16:1484–93.
Iimoto S, Watanabe S, Takahashi T, Shimizu A, Yamamoto H. The influence of Celecoxib on matrix synthesis by chondrocytes under mechanical stress in vitro. Int J Mol Med. 2005;16:1083–8.
Kourkoulis P, Michalopoulos G, Katifelis H, Giannopoulou I, Lazaris AC, Papaconstantinou I, et al. Leucine-rich alpha-2 glycoprotein 1, high mobility group box 1, matrix metalloproteinase 3 and annexin A1 as biomarkers of ulcerative colitis endoscopic and histological activity. Eur J Gastroenterol Hepatol. 2020;32(9):1106–15.
Tian Y, Xu J, Li Y, Zhao R, Du S, Lv C, et al. MicroRNA-31 reduces inflammatory signaling and promotes regeneration in colon epithelium, and delivery of mimics in microspheres reduces colitis in mice. Gastroenterol. 2019;156(8):2281–96 e.
Shinzaki S, Matsuoka K, Iijima H, Mizuno S, Serada S, Fujimoto M, et al. Leucine-rich alpha-2 glycoprotein is a serum biomarker of mucosal healing in ulcerative. J Crohns Colitis. 2017;11(1):84–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statement of Animal Rights
The in vivo trials were carried out based on the guidelines established by the Animal Care and use Committee of October 6 University, Giza, Egypt.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sheta, N.M., Boshra, S.A. Fabrication and Evaluation of Celecoxib Oral Oleogel to Reduce the Inflammation of Ulcerative Colitis. AAPS PharmSciTech 22, 180 (2021). https://doi.org/10.1208/s12249-021-02042-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02042-6