Abstract
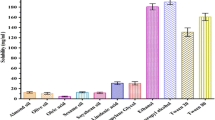
The current study aims at formulating and optimizing lipospheres (LS) by the Box-Behnken design (BBD) from safe biodegradable carnauba wax (CW) to co-administer saxagliptin (SG) and enalapril (EP) for co-existing chronic hypertensive diabetes in order to overcome inadequacies of conventional modes of drug administration. Optimized liposphere formulation (OLF) was selected by a numerical optimization procedure and a comparative in vivo pharmacokinetic study of OLF and commercial brands was also performed. Discrete, free-flowing, spherical, smooth-surface LS having a size range of 5–10 μm and zeta potential of − 20 to − 30 mV were successfully formulated. Compatibility studies by FTIR and DSC proved the lack of interaction of components while XRD suggested the transformation of crystalline drugs to amorphous form. Outcomes of dependent optimizing variables like percentage yield (30–90%), EP-release (32–92%), and SG-release (28–95%) followed a polynomial quadratic model. Pharmacokinetics studies indicated a significantly lower Cmax of EP (125.22 ± 6.32) and SG (75.63 ± 3.85) and higher mean Tmax values (9.4 h for EP and 10.73 h for SG) from OLF in comparison with reference brands of EP (257.54 ± 8.23 ng/mL) and SG (393.66 ± 2.97 ng/mL). Additionally, a potential rise in half-life and MRT of SG and EP was achieved reaching approximately 2- to 3-fold higher than noted for reference brands. Importantly, the enhanced Tmax and AUC0–24 specified the achievement of enhanced bioavailability of both drugs from LS. Consequently, such an innovative approach could not only control drug release in both in vitro and in vivo analyses but also maintain plasma drug concentration for a longer time without maximizing Cmax leading towards effective management of chronic illnesses.








Similar content being viewed by others
References
Messerli FH, Grossman E, Goldbourt U. Antihypertensive therapy in diabetic hypertensive patients. Am J Hypertens. 2001;14(S2):12S–6S.
Contreras F, Rivera M, Vasquez J, De la Parte MA, Velasco M. Diabetes and hypertension pathophysiology and therapeutics. J Hum Hypertens. 2000;14:S26–31.
Benford M, Milligan G, Pike J, Anderson P, Piercy J, Fermer S. Fixed-dose combination antidiabetic therapy: real-world factors associated with prescribing choices and relationship with patient satisfaction and compliance. Adv Ther. 2012;29(1):26–40.
Chobanian AV. Impact of nonadherence to antihypertensive therapy. Circulation. 2009;120:1558–60.
Shivakumar H, Patel P, Desai B, Ashok P, Arulmozhi S. Design and statistical optimization of glipizide loaded lipospheres using response surface methodology. Acta Pharma. 2007;57(3):269–85.
Whalen KL, Stewart RD. Pharmacologic management of hypertension in patients with diabetes. Am Fam Physician. 2008;78(11):1277–82.
Arauz-Pacheco C, Parrott MA, Raskin P. The treatment of hypertension in adult patients with diabetes (technical review). Diabetes Care. 2002;25:134–47.
Boulton DW. Clinical pharmacokinetics and pharmacodynamics of saxagliptin, a dipeptidyl peptidase-4 inhibitor. Clin Pharmacokinet. 2017;56(1):11–24.
Momoh M, Kenechukwu F, Attama A. Formulation and evaluation of novel solid lipid microparticles as a sustained release system for the delivery of metformin hydrochloride. Drug deliv. 2013;20(3–4):102–11.
Hao J, Fang X, Zhou Y, Wang J, Guo F, Li F, et al. Development and optimization of solid lipid nanoparticle formulation for ophthalmic delivery of chloramphenicol using a box-Behnken design. Int J Nanomedicine. 2011;6:683–92.
Jones E, Ojewole E, Kalhapure R, Govender T. In vitro comparative evaluation of monolayered multipolymeric films embedded with didanosine-loaded solid lipid nanoparticles: a potential buccal drug delivery system for ARV therapy. Drug Dev Ind Pharm. 2014;40(5):669–79.
Nemati E, Mokhtarzadeh A, Panahi-Azar V, Mohammadi A, Hamishehkar H, Mesgari-Abbasi M, et al. Ethambutol-loaded solid lipid nanoparticles as dry powder inhalable formulation for tuberculosis therapy. AAPS PharmSciTech. 2019;20:120.
Mohamed RA, Abass HA, Attia MA, Heikal OA. Formulation and evaluation of metoclopramide solid lipid nanoparticles for rectal suppository. J Pharm Pharmacol. 2013;65(11):1607–21.
Jaspart S, Piel G, Delattre L, Evrard B. Solid lipid microparticles: formulation, preparation, characterization, drug release and applications. Expert Opin Drug Del. 2005;2(1):75–87.
Kurade VP, Pai MG, Gude R. RP-HPLC estimation of Ramipril and Telmisartan in tablets. Indian J Pharm Sci. 2009;71(2):148–51.
Karthik A, Subramanian G, Mallikariuna RC, Krishnamurthy B, Ranjithkumar A, Musmade P, et al. Simultaneous determination of pioglitazone and glimepiride in bluk drug and pharmaceutical dosage form by RP-HPLC method. Pak J Pharm Sci. 2008;21(4):421–6.
Shazley GA. Ciprofloxacin controlled-solid lipid nanoparticles: Characterization, In-vitro release and antibacterial assessment. Biomed Res Int. 2017; Article ID:2120734.
Passerini N, Perissutti B, Albertini B, Voinovich D, Moneghini M, Rodriguez L. Controlled release of verapamil hydrochloride from waxy microparticles prepared by spray congealing. J Control Release. 2003;88(2):263–75.
Nandy BC, Mazumder B. Formulation and characterizations of delayed release multi- particulates system of indomethacin: optimization by response surface methodology. Curr Drug Deliv. 2014;11(1):72–86.
Jelvehgari M, Valizadeh H, Motlagh RJ, Montazam H. Formulation and physicochemical characterization of buccoadhesive microspheres containing diclofenac sodium. Adv Pharm Bull. 2014;4(3):295–301.
Dai W, Zhang D, Duan C, Jia L, Wang Y, Feng F, et al. Preparation and characteristics of oridonin-loaded nanostructured lipid carriers as a controlled-release delivery system. J Microencapsul. 2010;27(3):234–41.
Elsayed I, Abdelbary AA, Elshafeey HA. Nanosizing of a poorly soluble drug: technique optimization, factorial analysis, and pharmacokinetic study in healthy human volunteers. Int J Nanomedicine. 2014;9:2943–53.
Wu XG, Li G, Gao YL. Optimization of the preparation of nalmefene-loaded sustained- release microspheres using central composite design. Chem Pharm Bull. 2006;54(7):977–81.
Varshosaz J, Keihanfar M. Development and evaluation of sustained-release propranolol wax microspheres. J Microencapsul. 2001;18(3):277–84.
Srikanth MV, Rao NS, Sunil SA, Ram BJ, Kolapalli VRM. Statistical design and evaluation of a propranolol HCl gastric floating tablet. Acta Pharm Sin B. 2012;2(1):60–9.
Barakat NS, Yassin AEB. In vitro characterization of carbamazepine-loaded precifac lipospheres. Drug Deliv. 2006;13(2):95–104.
Vivek K, Reddy H, Murthy RSR. Investigations of effect of lipid matrix on drug entrapment, in vitro release and physical stability of olanzapine-loaded solid lipid nanoparticles. AAPS PharmSciTech. 2007;8:16–24.
Iswariya VT, Rao AHP, Babu VL, Rao AS. Formulation and evaluation of oro-dispersible tablets of saxagliptin. Int J Pharm Sci Rev Res. 2015;42:230–4.
Abbas AK, Alhamdany AT. Floating microspheres of enalapril maleate as a developed controlled release dosage form: investigation the effect of ionotropic gelation technique. Turk J Pharm Sci. 2018. https://doi.org/10.4274/tjps.15046.
Singh C, Koduri LVSK, Bhatt TD, Jhamb SS, Mishra V, Gill MS, et al. In vitro-in vivo evaluation of novel co-spray dried rifampicin phospholipid Lipospheres for Oral delivery. AAPS PharmSciTech. 2017;18:138–46.
Gomaa Y, Darwish I, Boraei N, El-Khordagui L. Formulation of wax oxybenzone microparticles using a factorial approach. J Microencapsul. 2010;27(7):628–39.
Milak S, Medlicott N, Tucker IG. Solid lipid microparticles containing loratadine prepared using a micromixer. J Microencapsul. 2006;23(8):823–31.
Mehnert W, Mader K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2):165–96.
Banerjee A, Roychoudhury J, Ali N. Stearylamine-bearing cationic liposomes kill leishmania parasites through surface exposed negatively charged phosphatidylserine. J Antimicrob Chemother. 2008;61:103–10.
Gowda D, Shivakumar H. Preparation and evaluation of waxes/fat microspheres loaded with lithium carbonate for controlled release. Indian J Pharm Sci. 2007;69(2):251.
Blychert E, Wingstrand K, Edgar B, Lidman K. Plasma concentration profiles and antihypertensive effect of conventional and extended-release felodipine tablets. Br J Clin Pharmacol. 1990;29(1):39–45.
Soma D, Attari Z, Reddy MS, Damodaram A, Koteshwara KB. Solid lipid nanoparticles of irbesartan: preparation, characterization, optimization and pharmacokinetic studies. Braz J Pharm Sci. 2017;53(1):e15012.
Momoh MA, Kenechukwu FC, Gwarzo MS, Builders PF. Formulation and evaluation of ibuprofen loaded lipospheres for effective oral drug delivery. Dhaka Univ J Pharm Sci. 2015;14(1):17–27.
Mahajan A, Kaur S. Design, characterization and pharmacokinetic studies of solid lipid nanoparticles of antihypertensive drug Telmisartan. Int J Pharm Sci Res. 2017;8(8):3402–12.
El-Say KM, Hosny KM. Optimization of carvedilol solid lipid nanoparticles: an approach to control the release and enhance the oral bioavailability on rabbits. PLoS One. 2018;13(8):e0203405.
Harde H, Das M, Jain S. Solid lipid nanoparticles: an oral bioavailability enhancer vehicle. Expert Opin Drug Deliv. 2011;8:1407–24.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declared that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maheen, S., Rasul, A., Hanif, M. et al. Lipospheres for Simultaneous Controlled Release and Improved Pharmacokinetic Profiles of Saxagliptin-Enalapril: Formulation, Optimization, and Comparative In Vitro-In Vivo Evaluation. AAPS PharmSciTech 21, 188 (2020). https://doi.org/10.1208/s12249-020-01733-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01733-w