Abstract
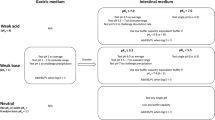
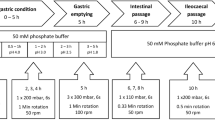
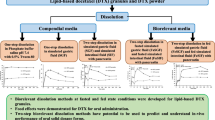
Dissolution testing and solubility determinations in different biorelevant media have gained considerable interest in the pharmaceutical industry from early-stage development of new products to forecasting bioequivalence. Among all biorelevant fluids, the preparation of fed-state simulated gastric fluid (FeSSGF) and handling of samples from dissolution/solubility testing in FeSSGF is considered to be relatively challenging. Challenges include maintaining the stability of FeSSGF medium upon sampling, filtration, and mitigating analytical interference of excipients and milk components. To overcome these challenges, standard and uniform working practices are required that are not only helpful in preparation of stable FeSSGF but also serve as a harmonizing guide for the collection of dissolution/solubility samples and their subsequent processing (i.e., handling and assay). The optimization of sample preparation methodology is crucial to reduce method-related variance by ensuring specificity, robustness, and reproducibility with acceptable recovery of the analytes. The sample preparation methodology includes a combination of techniques including filtration, solvent treatment, and centrifugation to remove the interfering media-related components and excipients from the analyte. The analytes of interest were chromatographically separated from the interfering analytes to quantify the drug concentration using the new high-performance liquid chromatography methods with ultraviolet detection. The methods developed allow rapid sample preparation, acceptable specificity, reproducible recoveries (greater than 95% of label claim), and quantification of study drugs (ibuprofen and ketoconazole). The sample preparation technique and method considerations provided here for ibuprofen and ketoconazole can serve as a starting point for solubility and dissolution testing of other small molecules in FeSSGF.







Similar content being viewed by others
References
Williams HD, Trevaskis NL, Charman SA, Shanker RM, Charman WN, Pouton CW, et al. Strategies to address low drug solubility in discovery and development. Pharmacol Rev. 2013;65(1):315–499.
Di L, Fish PV, Mano T. Bridging solubility between drug discovery and development. Drug Discov Today. 2012;17(9–10):486–95.
Di L, Kerns EH, Carter GT. Drug-like property concepts in pharmaceutical design. Curr Pharm Des. 2009;15(19):2184–94.
Klein S. The use of biorelevant dissolution media to forecast the in vivo performance of a drug. AAPS J. 2010;12(3):397–406.
Dressman JB, Reppas C. In vitro–in vivo correlations for lipophilic, poorly water-soluble drugs. Eur J Pharm Sci. 2000;11:S73–80.
Bou-Chacra N, Melo KJC, Morales IAC, Stippler ES, Kesisoglou F, Yazdanian M, et al. Evolution of choice of solubility and dissolution media after two decades of biopharmaceutical classification system. AAPS J. 2017;19(4):989–1001.
Bhagat NB. A review on development of biorelevant dissolution medium. Journal of Drug Delivery and Therapeutics. 2014;4(2):140–8.
Nicolaides E, Symillides M, Dressman JB, Reppas C. Biorelevant dissolution testing to predict the plasma profile of lipophilic drugs after oral administration. Pharm Res. 2001;18(3):380–8.
Vertzoni M, Dressman J, Butler J, Hempenstall J, Reppas C. Simulation of fasting gastric conditions and its importance for the in vivo dissolution of lipophilic compounds. Eur J Pharm Biopharm. 2005;60(3):413–7.
Shono Y, Jantratid E, Janssen N, Kesisoglou F, Mao Y, Vertzoni M, et al. Prediction of food effects on the absorption of celecoxib based on biorelevant dissolution testing coupled with physiologically based pharmacokinetic modeling. Eur J Pharm Biopharm. 2009;73(1):107–14.
Takács-Novák K, Szőke V, Völgyi G, Horváth P, Ambrus R, Szabó-Révész P. Biorelevant solubility of poorly soluble drugs: rivaroxaban, furosemide, papaverine and niflumic acid. J Pharm Biomed Anal. 2013;83:279–85.
Kalantzi L, Goumas K, Kalioras V, Abrahamsson B, Dressman JB, Reppas C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm Res. 2006;23(1):165–76.
Fang JB, Robertson VK, Rawat A, Flick T, Tang ZJ, Cauchon NS, et al. Development and application of a biorelevant dissolution method using USP apparatus 4 in early phase formulation development. Mol Pharm. 2010;7(5):1466–77.
Galia E, Nicolaides E, Hörter D, Löbenberg R, Reppas C, Dressman J. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm Res. 1998;15(5):698–705.
Jantratid E, De Maio V, Ronda E, Mattavelli V, Vertzoni M, Dressman JB. Application of biorelevant dissolution tests to the prediction of in vivo performance of diclofenac sodium from an oral modified-release pellet dosage form. Eur J Pharm Sci. 2009;37(3–4):434–41.
Sunesen VH, Pedersen BL, Kristensen HG, Müllertz A. In vivo in vitro correlations for a poorly soluble drug, danazol, using the flow-through dissolution method with biorelevant dissolution media. Eur J Pharm Sci. 2005;24(4):305–13.
Jantratid E, Dressman J. Biorelevant dissolution media simulating the proximal human gastrointestinal tract: an update. Dissolut Technol. 2009;16(3):21–5.
Early R. Dairy products and milk-based food ingredients. Natural food additives, ingredients and flavourings: Elsevier; 2012. p. 417–45.
Klein S, Dressman JB, Butler J, Hempenstall JM, Reppas C. Media to simulate the postprandial stomach. I. Matching the physicochemical characteristics of standard breakfasts. J Pharm Pharmacol. 2004;56(5):605–10.
Cluskey F, Thomas E, Coulter S. Precipitation of milk proteins by sodium carboxymethylcellulose1. J Dairy Sci. 1969;52(8):1181–5.
Maubois J. Separation, extraction and fractionation of milk protein components. Lait. 1984;64(645–646):485–95.
Fríguls B, Joya X, García-Algar O, Pallás C, Vall O, Pichini S. A comprehensive review of assay methods to determine drugs in breast milk and the safety of breastfeeding when taking drugs. Anal Bioanal Chem. 2010;397(3):1157–79.
Diakidou A, Vertzoni M, Dressman J, Reppas C. Estimation of intragastric drug solubility in the fed state: comparison of various media with data in aspirates. Biopharm Drug Dispos. 2009;30(6):318–25.
Shah H, Shah V, Parikh D, Butani S, Mehta T. Dissolution improvement of nebivolol hydrochloride using solid dispersion adsorbate technique. Asian Journal of Pharmaceutics (AJP): Free full text articles from Asian J Pharm. 2015;9(1):49–55.
Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, et al. PubChem substance and compound databases. Nucleic Acids Res. 2015;44(D1):D1202–D13.
Davanzo R, Bua J, Paloni G, Facchina G. Breastfeeding and migraine drugs. Eur J Clin Pharmacol. 2014;70(11):1313–24.
Gillespie W, DiSanto A, Monovich R, Albert K. Relative bioavailability of commercially available ibuprofen oral dosage forms in humans. J Pharm Sci. 1982;71(9):1034–8.
Huang Y, Colaizzi J, Bierman R, Woestenborghs R, Heykants J. Pharmacokinetics and dose proportionality of ketoconazole in normal volunteers. Antimicrob Agents Chemother. 1986;30(2):206–10.
ICH Q2 (R1): validation of analytical procedures: text and methodology. International Conference on Harmonization, Geneva; 2005.
United States Pharmacopeia, USP 39-NF34. General chapter on the dissolution procedure: development and validation <1092>. United States Pharmacopeial Convention Rockville, MD; 2016.
United States Pharmacopeia, USP 39–NF34. General chapter on validation of compendial procedures < 1225>. United States Pharmacopeial Convention Rockville, MD; 2016.
Crawford Scientific. The theory of HPLC chromatographic parameters. Available from https://www.chromacademy.com/lms/sco2/Theory_Of_HPLC_Chromatographic_Parameters.pdf.
Dressman JB, Amidon GL, Reppas C, Shah VP. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm Res. 1998;15(1):11–22.
Vargaftik N, Volkov B, Voljak L. International tables of the surface tension of water. J Phys Chem Ref Data. 1983;12(3):817–20.
Ghazal HS, Dyas AM, Ford JL, Hutcheon GA. The impact of food components on the intrinsic dissolution rate of ketoconazole. Drug Dev Ind Pharm. 2015;41(10):1647–54.
United States Food and Drug Administration. Guidance for industry - bioanalytical method validation. Available from https://wwwfdagov/downloads/drugs/guidances/ucm070107pdf. 2018. Accessed 30 Nov 2019.
Savu SN, Silvestro L, Mircioiu C, Anuta V. Development of in vitro in vivo correlation models for clopidogrel tablets to describe administration under fasting and fed conditions. Therapy. 2016;11(16):18.
Farrar H, Letzig L, Gill M. Validation of a liquid chromatographic method for the determination of ibuprofen in human plasma. J Chromatogr B. 2002;780(2):341–8.
Velikinac I, Čudina O, Janković I, Agbaba D, Vladimirov S. Comparison of capillary zone electrophoresis and high performance liquid chromatography methods for quantitative determination of ketoconazole in drug formulations. Il Farmaco. 2004;59(5):419–24.
Valko K, Nunhuck S, Bevan C, Abraham MH, Reynolds DP. Fast gradient HPLC method to determine compounds binding to human serum albumin. Relationships with octanol/water and immobilized artificial membrane lipophilicity. J Pharm Sci. 2003;92(11):2236–48.
Jantratid E, Janssen N, Reppas C, Dressman JB. Dissolution media simulating conditions in the proximal human gastrointestinal tract: an update. Pharm Res. 2008;25(7):1663–76.
Jantratid E, Janssen N, Chokshi H, Tang K, Dressman JB. Designing biorelevant dissolution tests for lipid formulations: case example–lipid suspension of RZ-50. Eur J Pharm Biopharm. 2008;69(2):776–85.
Levis KA, Lane ME, Corrigan OI. Effect of buffer media composition on the solubility and effective permeability coefficient of ibuprofen. Int J Pharm. 2003;253(1–2):49–59.
Disclaimer
The approaches and conclusions reported in this publication have not been formally disseminated by the US Food and Drug Administration and should not be construed to represent any Agency determination or policy.
Funding
We gratefully acknowledge the US Food and Drug Administration (USFDA) and the National Institute for Pharmaceutical technology and Education (NIPTE) for financial support. This study was funded by the FDA Grant to NIPTE titled “The Critical Path Manufacturing Sector Research Initiative (U01)”; Grant No. 5U01FD004275.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Ajaz S. Hussain, Kenneth Morris, and Vadim J. Gurvich
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shah, H.S., Sardhara, R., Nahar, K. et al. Development and Validation of Sample Preparation and an HPLC Analytical Method for Dissolution Testing in Fed-State Simulated Gastric Fluid—Illustrating Its Application for Ibuprofen and Ketoconazole Immediate Release Tablets. AAPS PharmSciTech 21, 172 (2020). https://doi.org/10.1208/s12249-020-01702-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01702-3