Abstract
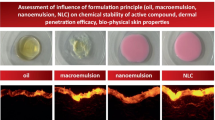
Idebenone (IDB) is a synthetic antioxidant and analog of coenzyme Q10. The percutaneous permeation of IDB was investigated in guinea pig skin after application of different formulations. The enhancing effects of various formulations [nanostructured lipid carriers (NLCs), nanoemulsion (NE), or oil solution] on the permeation of IDB were evaluated using ex vivo guinea pig skins. Furthermore, stability of different formulations and in which chemical stability of IDB was determined during storage. Permeation experiments revealed that formulations varied in their ability to enhance the skin permeation of IDB. For NLC formulation, the cumulative amount of IDB in the epidermis, dermis, and acceptor medium of diffusion cells was approximately threefold more than NE or oil solution at the end of 24-h experiment. No significant difference between NE and oil solution was observed in the enhancement of penetration efficacy of IDB. Different formulations resulted in stability with different properties. NLC formulation revealed preferentially more stable than NE. The residual percentage of IDB loaded in NLCs, NE, and oil solution was 90.1%, 65.4%, and 51.3%, respectively, when stored at 40°C under 75% RH and 3,000 lx light conditions for 180 days. The results obtained here demonstrated that the abilities of NLCs to improve the chemical stability of IDB and enhance the skin permeation are much better than NE and oil solution. These suggest that NLCs containing IDB have significant potential use for skin care as an alternative topical formulation.



Similar content being viewed by others
REFERENCES
Mcdaniel DH, Neudecker BA, Dinardo JC, Lewis JA, Maibach H. Clinical efficacy assessment in photodamage skin of 0.5% and 1.0% idebenone. J Cosmet Dermatol. 2005;4:167–73.
Farris P. Idebenone, green tea, and Coffeeberry® extract: new and innovative antioxidants. Dermatol Ther. 2007;20:322–9.
McDaniel DH, Neudecker BA, DiNardo JC, Lewis JA, Maibach HI. Idebenone: a new antioxidant—Part I. Relative assessment of oxidative stress protection capacity compared to commonly known antioxidants. J Cosmet Dermatol. 2005;4:10–7.
Amorim CM, Couto AG, Netz DJA, de Freitas RA, Bresolin TMB. Antioxidant idebenone-loaded nanoparticles based on chitosan and N-carboxymethylchitosan. Nanomedicine. 2010;6:745–52.
Palumbo M, Russo A, Cardile V, Renis M, Paolino D, Puglis G, Fresta M. Improved antioxidant effect of idebenone-loaded polyethyl-2-cyanoacrylate nanocapsules tested on human fibroblasts. Pharm Res. 2002;19:71–8.
Amidi M, Mastrobattista E, Jiskoot W, Hennink WH. Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev. 2010;62:59–82.
Müller RH, Petersen RD, Hommoss A, Pardeike J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv Drug Deliv Rev. 2007;59:522–30.
Obeidat WM, Schwabe K, Müller RH, Keck CM. Preservation of nanostructured lipid carriers (NLC). Eur J Pharm Biopharm. 2010;76:56–67.
Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–96.
Chen CC, Tsai TH, Huang ZR, Fang JY. Effects of lipophilic emulsifiers on the oral administration of lovastatin from nanostructured lipid carriers: physicochemical characterization and pharmacokinetics. Eur J Pharm Biopharm. 2010;74:474–82.
Artuch R, Colomé C, Vilaseca MA, Aracil A, Pineda M. Monitoring of idebenone treatment in patients with Friedreich’s ataxia by high-pressure liquid chromatography with electrochemical detection. J Neurosci Meth. 2002;115:63–6.
Fang JY, Fang CL, Liu CH, Su YH. Lipid nanoparticles as vehicles for topical psoralen delivery: solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC). Eur J Pharm Biopharm. 2008;70:633–40.
Gillet A, Compre P, Lecomte F, Hubert P, Ducat E, Evrard B, et al. Liposome surface charge influence on skin penetration behaviour. Int J Pharm. 2011;411:223–31.
Junyaprasert VB, Teeranachaideekul V, Souto EB, Boonme P, Müller RH. Q10-loaded NLC versus nanoemulsions: stability, rheology and in vitro skin permeation. Int J Pharm. 2009;377:207–14.
Driscoll D. Lipid injectable emulsions: pharmacopeial and safety issues. Pharm Res. 2006;23:1959–69.
Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54:131–55.
Peltola S, Saarinen-Savolainen P. Microemulsions for topical delivery of estradiol. Int J Pharm. 2003;254:99–107.
Freitas C, Müller RH. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN™) dispersions. Int J Pharm. 1998;168:221–9.
Pathak P, Nagarsenker M. Formulation and evaluation of lidocaine lipid nanosystems for demal delivery. AAPS PharmSciTech. 2009;10:985–92.
Tursilli R, Casolari A, Iannuccelli V, Scalia S. Enhancement of melatonin photostability by encapsulation in lipospheres. J Pharmaceut Biomed. 2006;40:910–4.
Mordente A, Martorana GE, Minotti G, Giardina B. Antioxidant properties of 2,3-dimethoxy-5-methyl-6-(10-hydroxydecyl)-1,4- benzoquinone (Idebenone). Chem Res Toxicol. 1998;11:54–63.
Alvarez-Roman R, Naik A, Kalia YN, Guy RH, Fessi H. Skin penetration and distribution of polymeric nanoparticles. J Control Release. 2004;99:53–62.
Rouse JG, Yang J, Ryman-Rasmussen JP, Barron AR, Monteiro-Riviere NA. Effects of mechanical flexion on the penetration of fullerene amino acid-derivatized peptide nanoparticles through skin. Nano Lett. 2007;7:155–60.
Singh P, Sihorkar V, Jaitely V, Kanaujia P, Vyas SP. Pilosebaceous unit: anatomical considerations and drug delivery opportunities. Ind J Pharmacol. 2000;32:269–81.
Lademann J, Richter H, Schanzer S, Knorr F, Meinke M, Sterry W, et al. Penetration and storage of particles in human skin: perspectives and safety aspects. Eur J Pharm Biopharm. 2011;77:465–8.
Toll R, Jacobi U, Richter H, Lademann J, Schaefer H, Blume-Peytavi U. Penetration profile of microspheres in follicular targeting of terminal hair follicles. J Invest Dermatol. 2004;123:168–76.
Lademann J, Richter H, Teichmann A, Otberg N, Blume-Peytavi U, Luengo J, et al. Nanoparticles—an efficient carrier for drug delivery into the hair follicles. Eur J Pharm Biopharm. 2007;66:159–64.
Nam SH, Ji XY, Park JS. Investigation of tacrolimus loaded nanostructured lipid carriers for topical drug delivery. Bull Korean Chem Soc. 2011;32:956–60.
Nokhodchi A, Shokri J, Dashbolaghi A, Hassan-Zadeh D, Ghafourian T, Barzegar-Jalali M. The enhancement effect of surfactants on the penetration of lorazepam through rat skin. Int J Pharm. 2003;250:359–69.
Bos JD, Meinardi MMHM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9:165–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, B., Ge, ZQ. Nanostructured Lipid Carriers Improve Skin Permeation and Chemical Stability of Idebenone. AAPS PharmSciTech 13, 276–283 (2012). https://doi.org/10.1208/s12249-011-9746-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-011-9746-3