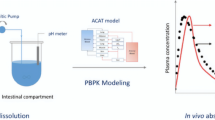
Abstract
The present work aimed to differentiate between in vitro dissolution profiles of ibuprofen as input for GastroPlus™ and to see the impact on systemic exposure. In vitro dissolution profiles of ibuprofen obtained under low- and high-buffered dissolution media were used as input using the z-factor approach. In a second step, a customized surface pH calculator was applied to predict the surface pH of ibuprofen under these low- and high-buffered dissolution conditions. These surface pH values were adopted in GastroPlus™ and simulations were performed to predict the systemic outcome. Simulated data were compared with systemic data of ibuprofen obtained under fasted state conditions in healthy subjects. The slower dissolution rate observed when working under low-buffered conditions nicely matched with the slower dissolution rate as observed during the clinical aspiration study and was in line with the systemic exposure of the drug. Finally, a population simulation was performed to explore the impact of z-factor towards bioequivalence (BE) criteria (so-called safe space). Concerning future perspectives, the customized calculator should be developed in such a way to make it possible to predict the dissolution rate (being informed by the particle size distribution) which, in its turn, can be used as a surrogate to predict the USP2 dissolution curve. Subsequently, validation can be done by using this profile as input for PBPK platforms.
Graphical Abstract






Similar content being viewed by others
Abbreviations
- API:
-
Active pharmaceutical ingredient
- BE:
-
Bioequivalence
- CI:
-
Confidence interval
- CV:
-
Coefficient of variation
- FDA:
-
Food and Drug Administration
- GI:
-
Gastrointestinal
- IR:
-
Immediate-release
- PBPK:
-
Physiologically based pharmacokinetic modeling
- PSA:
-
Parameter sensitivity analysis
References
US Food & Drug Administration. The use of physiologically based pharmacokinetic analyses — biopharmaceutics applications for oral drug product development, manufacturing changes, and controls [Internet]. U.S. Food and Drug Administration. FDA; 2020 [cited 2021 Jun 8]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-physiologically-based-pharmacokinetic-analyses-biopharmaceutics-applications-oral-drug-product
Kostewicz ES, Abrahamsson B, Brewster M, Brouwers J, Butler J, Carlert S, et al. In vitro models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2014;57:342–66.
Butler J, Hens B, Vertzoni M, Brouwers J, Berben P, Dressman J, et al. In vitro models for the prediction of in vivo performance of oral dosage forms: recent progress from partnership through the IMI OrBiTo collaboration. Eur J Pharm Biopharm. 2019;136:70–83.
Hens B, Corsetti M, Spiller R, Marciani L, Vanuytsel T, Tack J, et al. Exploring gastrointestinal variables affecting drug and formulation behavior: methodologies, challenges and opportunities. Int J Pharm. 2016;59:79–97.
Amaral Silva D, Al-Gousous J, Davies NM, Bou Chacra N, Webster GK, Lipka E, et al. Simulated, biorelevant, clinically relevant or physiologically relevant dissolution media: the hidden role of bicarbonate buffer. Eur J Pharm Biopharm. 2019;142:8–19.
Vertzoni M, Diakidou A, Chatzilias M, Söderlind E, Abrahamsson B, Dressman JB, et al. Biorelevant media to simulate fluids in the ascending colon of humans and their usefulness in predicting intracolonic drug solubility. Pharm Res. 2010;27:2187–96.
Dressman JB, Amidon GL, Reppas C, Shah VP. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm Res. 1998;15:11–22.
Hofmann M, Thieringer F, Nguyen MA, Månsson W, Galle PR, Langguth P. A novel technique for intraduodenal administration of drug suspensions/solutions with concurrent pH monitoring applied to ibuprofen formulations. Eur J Pharm Biopharm. 2019;136:192–202.
Cristofoletti R, Dressman JB. Matching phosphate and maleate buffer systems for dissolution of weak acids: equivalence in terms of buffer capacity of bulk solution or surface pH? Eur J Pharm Biopharm. 2016;103:104–8.
Mooney KG, Mintun MA, Himmelstein KJ, Stella VJ. Dissolution kinetics of carboxylic acids II: effect of buffers. J Pharm Sci. 1981;70:22–32.
Mooney KG, Mintun MA, Himmelstein KJ, Stella VJ. Dissolution kinetics of carboxylic acids I: effect of pH under unbuffered conditions. J Pharm Sci. 1981;70:13–22.
Ozturk SS, Palsson BO, Dressman JB. Dissolution of ionizable drugs in buffered and unbuffered solutions. Pharm Res. 1988;5:272–82.
Hens B, Sinko PD, Job N, Dean M, Al-Gousous J, Salehi N, et al. Formulation predictive dissolution (fPD) testing to advance oral drug product development: an introduction to the US FDA funded “21st Century BA/BE” project. Int J Pharm. 2018;548:120–7.
Augustijns P, Vertzoni M, Reppas C, Langguth P, Lennernäs H, Abrahamsson B, et al. Unraveling the behavior of oral drug products inside the human gastrointestinal tract using the aspiration technique: history, methodology and applications. Eur J Pharm Sci. 2020;155:105517.
Litou C, Psachoulias D, Vertzoni M, Dressman J, Reppas C. Measuring pH and buffer capacity in fluids aspirated from the fasted upper gastrointestinal tract of healthy adults. Pharm Res. 2020;37:42.
Hens B, Tsume Y, Bermejo M, Paixao P, Koenigsknecht MJ, Baker JR, et al. Low buffer capacity and alternating motility along the human gastrointestinal tract: implications for in vivo dissolution and absorption of ionizable drugs. Mol Pharm. 2017;14:4281–94.
Fadda HM, Sousa T, Carlsson AS, Abrahamsson B, Williams JG, Kumar D, et al. Drug solubility in luminal fluids from different regions of the small and large intestine of humans. Mol Pharmaceutics. 2010;7:1527–32.
Bermejo M, Paixão P, Hens B, Tsume Y, Koenigsknecht MJ, Baker JR, et al. Linking the gastrointestinal behavior of ibuprofen with the systemic exposure between and within humans-part 1: fasted state conditions. Mol Pharm. 2018;15:5454–67.
Koenigsknecht MJ, Baker JR, Wen B, Frances A, Zhang H, Yu A, et al. In vivo dissolution and systemic absorption of immediate release ibuprofen in human fastrointestinal tract under fed and fasted conditions. Mol Pharm. 2017;14:4295–304.
Al-Gousous J, Salehi N, Amidon GE, Ziff RM, Langguth P, Amidon GL. Mass transport analysis of bicarbonate buffer: effect of the CO2-H2CO3 hydration-dehydration kinetics in the fluid boundary layer and the apparent effective p Ka controlling dissolution of acids and bases. Mol Pharm. 2019;16:2626–35.
Hofmann M, García MA, Al-Gousous J, Ruiz-Picazo A, Thieringer F, Nguyen MA, et al. In vitro prediction of in vivo absorption of ibuprofen from suspensions through rational choice of dissolution conditions. Eur J Pharm Biopharm. 2020;149:229–37.
Krieg BJ, Taghavi SM, Amidon GL, Amidon GE. In vivo predictive dissolution: transport analysis of the CO2, bicarbonate in vivo buffer system. J Pharm Sci. 2014;103:3473–90.
Krieg BJ, Taghavi SM, Amidon GL, Amidon GE. In vivo predictive dissolution: comparing the effect of bicarbonate and phosphate buffer on the dissolution of weak acids and weak bases. J Pharm Sci. 2015;104:2894–904.
Tsume Y, Langguth P, Garcia-Arieta A, Amidon GL. In silico prediction of drug dissolution and absorption with variation in intestinal pH for BCS class II weak acid drugs: ibuprofen and ketoprofen. Biopharm Drug Dispos. 2012;33:366–77.
Bermejo M, Hens B, Dickens J, Mudie D, Paixão P, Tsume Y, et al. A mechanistic physiologically-based biopharmaceutics modeling (PBBM) approach to assess the in vivo performance of an orally administered drug product: from IVIVC to IVIVP. Pharmaceutics. 2020;12.
Augustijns P, Wuyts B, Hens B, Annaert P, Butler J, Brouwers J. A review of drug solubility in human intestinal fluids: implications for the prediction of oral absorption. Eur J Pharm Sci. 2014;57:322–32.
Yu A, Koenigsknecht MJ, Hens B, Baker JR, Wen B, Jackson TL, et al. Mechanistic deconvolution of oral absorption model with dynamic gastrointestinal fluid to predict regional rate and extent of GI drug dissolution. AAPS J. 2019;22:3.
Takano R, Sugano K, Higashida A, Hayashi Y, Machida M, Aso Y, et al. Oral absorption of poorly water-soluble drugs: computer simulation of fraction absorbed in humans from a miniscale dissolution test. Pharm Res. 2006;23:1144–56.
Pavliv L, Voss B, Rock A. Pharmacokinetics, safety, and tolerability of a rapid infusion of i.v. ibuprofen in healthy adults. Am J Health Syst Pharm. 2011;68:47–51.
Takano R, Kataoka M, Yamashita S. Integrating drug permeability with dissolution profile to develop IVIVC. Biopharm Drug Dispos. 2012;33:354–65.
Cristofoletti R, Hens B, Patel N, Esteban VV, Schmidt S, Dressman J. Integrating drug- and formulation-related properties with gastrointestinal tract variability using a product-specific particle size approach: case example ibuprofen. J Pharm Sci. 2019;108:3842–7.
Al-Gousous J, Amidon GL, Langguth P. Toward biopredictive dissolution for enteric coated dosage forms. Mol Pharm. 2016;13:1927–36.
Sheng JJ, McNamara DP, Amidon GL. Toward an in vivo dissolution methodology: a comparison of phosphate and bicarbonate buffers. Mol Pharm. 2009;6:29–39.
McNamara DP, Whitney KM, Goss SL. Use of a physiologic bicarbonate buffer system for dissolution characterization of ionizable drugs. Pharm Res. 2003;20:1641–6.
Sheng JJ, Kasim NA, Chandrasekharan R, Amidon GL. Solubilization and dissolution of insoluble weak acid, ketoprofen: effects of pH combined with surfactant. Eur J Pharm Sci. 2006;29:306–14.
Jede C, Wagner C, Kubas H, Weigandt M, Weber C, Lecomte M, et al. Improved prediction of in vivo supersaturation and precipitation of poorly soluble weakly basic drugs using a biorelevant bicarbonate buffer in a gastrointestinal transfer model. Mol Pharm. 2019;16:3938–47.
Fadda HM, Merchant HA, Arafat BT, Basit AW. Physiological bicarbonate buffers: stabilisation and use as dissolution media for modified release systems. Int J Pharm. 2009;382:56–60.
Hens B, Bermejo M, Tsume Y, Gonzalez-Alvarez I, Ruan H, Matsui K, et al. Evaluation and optimized selection of supersaturating drug delivery systems of posaconazole (BCS class 2b) in the gastrointestinal simulator (GIS): an in vitro-in silico-in vivo approach. Eur J Pharm Sci. 2018;115:258–69.
Verwei M, Minekus M, Zeijdner E, Schilderink R, Havenaar R. Evaluation of two dynamic in vitro models simulating fasted and fed state conditions in the upper gastrointestinal tract (TIM-1 and tiny-TIM) for investigating the bioaccessibility of pharmaceutical compounds from oral dosage forms. Int J Pharm. 2016;498:178–86.
Acknowledgements
The authors would like to thank the EPSRC for provision of a studentship on the Centre for Doctoral Training in Transformative Pharmaceutical Technologies (EP/S023054/1) to Nidhi Seegobin.
Funding
Data obtained from the ibuprofen clinical aspiration study were funded by the grant #HHSF223201510157C and #HHSF223201310144C by the US Food and Drug Administration (FDA).
Author information
Authors and Affiliations
Contributions
All authors were involved in the development of the manuscript, interpretation of data, and have read and approved the final version, and have met the criteria for authorship as established by the ICMJE.
Corresponding authors
Ethics declarations
Conflict of Interest
Bart Hens, Nicola Clear, and Mark McAllister are full-time employees of Pfizer UK. Yasuhiro Tsume is a full-time employee of Merck and Co., USA.
Additional information
Guest Editors: Rodrigo Cristofoletti and Lawrence Yu
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hens, B., Seegobin, N., Bermejo, M. et al. Dissolution Challenges Associated with the Surface pH of Drug Particles: Integration into Mechanistic Oral Absorption Modeling. AAPS J 24, 17 (2022). https://doi.org/10.1208/s12248-021-00663-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00663-0