Abstract
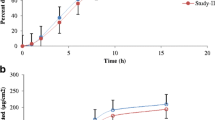
The purpose of this study was to construct a pharmacokinetic (PK) model and to determine PK parameters of 2,3,5,6-tetramethylpyrazine (TMP) after application of TMP transdermal delivery system. Data were obtained in Sprague-Dawley (SD) rats following a single dose of TMP transdermal delivery system. Blood samples were obtained at 0, 0.25, 0.5, 1, 2, 4, 6, 16, and 24 hours after the transdermal application. In the brain level study, 18 SD rats were divided into 6 groups. Three SD rats before and after transdermal application were culled and sacrificed at each of the following time intervals: 2, 4, 6, 16, and 24 hours after the TMP-TTS application. TMP concentrations in plasma and brain tissues were determined using high performance liquid chromatography and data were fitted using a zero-order absorption and a firstorder-elimination 3-compartment PK model. Fitted parameters included 2 volumes of distribution (V1, V2) and 2 elimination rate constants (k10, k20). The elimination half-life for TMP in plasma and brain was 26.5 and 31.2 minutes, respectively. The proposed PK model fit observed concentrations of TMP very well. This model is useful for predicting drug concentrations in plasma and brain and for assisting in the development of transdermal systems.
Similar content being viewed by others
References
Guo SK, Chen KJ, Qian ZH, Weng WL, Qian MY. Tetramethylpyrazine in the treatment of cardiovascular and cerebrovascular diseases. Planta Med. 1983;47:89.
Chen KJ, Chen K. Ischemic stroke treated with Ligusticum chuanxiong. Chin Med J (Engl) 1992;105(10):870–3.
Watanabe H. Candidates for cognitive enhancer extracted from medicinal plants: paeoniflorin and tetramethylpyrazine. Behav Brain Res. 1997;83:138–141.
Liang CC, Hong CY, Chen CF, Tsai TH. Measurement and pharmacokinetic study of tetramethylpyrazine in rat blood and its regional brain tissue by high-performance liquid chromatography. J Chromatogr B Biomed Sci Applic. 1999;724(2):303–309.
Tsai T, Liang C. Pharmacokinetics of tetramethylpyrazine in rat blood and brain using microdialysis. Int J Pharm. 2001;216(1–2):61–66.
Cai W, Dong SN, Lou YQ. HPLC determination of tetramethylpyrazine in human serum and its pharmacokinetic parameters [in Chinese with English abstract]. Acta Pharm Sinica. 1989;24(12):881–886.
Lou YQ, Zhang H, Cao X, Chen ML. The pharmacokinetics and disposition of tetramethylpyrazine phosphate in dogs and rats [in Chinese with English abstract]. Acta Pharm Sinica. 1986;21(7):481–487.
Sun BL. Clinical use of tetramethylpyrazine: a review [in Chinese] Chin J Integr Tradit West Med. 1994;14:465–468.
Jia WC. Gao Yao Fang Ji. Beijing, China: Peoples Public Health Publisher, 1957.
Wang GQ. Zhong Guo Gao Yao Xue. Xian, China: Science and Technology Publisher; 1981:111–154.
Brown L, Langer R. Transdermal delivery of drugs. Annu Rev Med. 1988;39:221–229.
Chen KJ March of Integration of TCM and WM Towards 21st Century [in Chinese]. Beijing, China: Medicine Pharmaceutics Science-Technology Publisher, 1991:207–216.
Guy RH, Hadgraft J. Selection of drug candidates for transdermal delivery. In: Hadgraft J, Guy RH, eds. Transdermal Drug Delivery: Development Issues and Research Initiatives. New York, NY: Marcel Dekker, 1989:59–81.
Flynn GL, Stewart B. Percutaneous drug penetration: choosing candidates for transdermal development. Drug Dev Res. 1988;13:169–185.
Qi XH, Hou HM. Transdermal delivery system of tetramethylpyrazine: the influence of varied EVA copolymer membrane on skin permeation of TMP. In: CRS-CPA Joint Workshop on Recent Advances in Drug Delivery Science and Technology, Beijing, September 20–22, China. 1997:48.
Qi XH. In Vitro and In Vivo Skin Permeation Study of Chuanxiong [dissertation]. Shanghai, China: Shanghai Institute of Industrial Pharmaceutics, 1999.
Matsuyama K, Nakashima M, Ichikawa M, Goto S. In vivo microdialysis for the transdermal absorption of valproate in rats. Biol Pharm Bull. 1994;17(10):1395–1398.
Matsuyama K, Nakashima M, Ichikawa M, Goto S. Application of in vivo microdialysis to transdermal absorption of methotrexate in rats. Pharm Res. 1994;11(5):684–686.
Bradley CR, Almirez RG, Conner DP, Rhyne PR, Peck CC. Noninvasive transdermal chemical collection, II: in vitro and in vivo skin permeability studies. Skin Pharmacol. 1990;3(4):227–235.
Ritschel W, Starzacher A., Sabouni A, Hussain AS, Koch HP. Percutaneous absorption of rosmarinic acid in the rat. Methods Find Exp Clin Pharmacol. 1989;11(5):345–352.
Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, NY: Marcel Dekker, 1982.
Tamai I, Tsuji A. Drug delivery through the blood-brain barrier. Adv Drug Deliv Rev. 1996;9(3):401–424.
Chen X, Dong SN. Studies on the metabolites of tetramethylpyrazine in rabbits [in Chinese with English abstract]. Acta Pharm Sinica. 1996;31(8):617–621.
Li M, Handa S, Ikeda Y, Goto S. Specific inhibiting characteristics of tetramethylpyrazine, one of the active ingredients of the Chinese herbal medicine Chuanxiong, on platelet thrombus formation under high shear rates. Thromb Res. 2001;104(1):15–28.
Win Nonlin, Version 2.1., Palo Alto, CA. Pharsight Corporation. 1994.
Guy RH, Hadgraft J. The prediction of plasma levels of drugs following transdermal application. J Control Release. 1985;1:177–182.
Guy RH, Hadgraft J. Interpretation and prediction of the kinetics of transdermal drug delivery: oestradiol, hyoscine, and timolol. Int J Pharm. 1986;32:159–163.
Watanabe H. Candidates for cognitive enhancer extracted from medicinal plants: paeoniflorin and tetramethylpyrazine. Behav Brain Res. 1997;83:138–141.
Author information
Authors and Affiliations
Additional information
Published: December 13, 2002
Rights and permissions
About this article
Cite this article
Qi, X., Ackermann, C., Sun, D. et al. The prediction of plasma and brain levels of 2,3,5,6-tetramethylpyrazine following transdermal application. AAPS J 4, 46 (2002). https://doi.org/10.1208/ps040446
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/ps040446