Abstract
The Sc2O3: Er3+, Yb3+ nanoparticles (NPs) with the size of about 19 nm were synthesized by a simple oleic acid-mediated hydrothermal (HT) process. X-ray diffraction (XRD), transmission electron microscopy (TEM), upconversion luminescence (UCL) spectra, and decay curves were used to characterize the resulting samples. The Sc2O3: Er3+, Yb3+ NPs made by HT method exhibit the stronger UCL, of which the red UCL are enhanced by a factor of 4, in comparison with those samples prepared by solvothermal (ST) method at the same optimized lanthanide ion concentrations. The UCL enhancement can be attributed to the reduced surface groups and longer lifetimes. Under 980 nm wavelength excitation, the decay curves of Er3+: (2H11/2, 4S3/2) → 4I15/2 and 4F9/2 → 4I15/2 emissions for Sc2O3: Er3+, Yb3+ NPs samples are both close to each other, resulting from the cross relaxation energy transfer from Er3+ to Yb3+, followed by an energy back transfer within the same Er3+-Yb3+ pair. Also, under the relatively low-power density, the slopes of the linear plots of log(I) vs. log(P) for red and green emissions are 2.5 and 2.1, implying the existence of three-photon processes. Our results indicate that Sc2O3: Er3+, Yb3+ NPs is an excellent material for achieving intense UCL with small size in the biological fields.
Similar content being viewed by others
Introduction
Infrared to visible upconversion luminescence (UCL) has been extensively studied for its fundamental value [1,2,3] and its various potential applications in upconversion lasers, bioimaging, infrared imaging, solar cells, etc. [4,5,6,7,8]. The co-doping of Er3+ and a high concentration of sensitizer Yb3+ forms the most attractive energy transfer (ET) upconversion system [1]. Under 980 nm infrared excitation of the sensitizer Yb3+, this system can generate green and red emission originating from the (2H11/2, 4S3/2) → 4I15/2 and 4F9/2 → 4I15/2 transitions of Er3+, respectively [9]. Selection of appropriate host material is essential in the synthesis of lanthanide-doped nanocrystals (NCs) with favorable optical properties such as high UC efficiency and controllable emission profile. The practical applications require the development of more efficient, high stability UC materials with low excitation density [10, 11]. Oxide materials are usually very stable chemically, mechanically, and thermally, and could therefore be promising hosts for UC applications [3, 12,13,14,15,16]. The cubic sesquioxide materials (such as Y2O3, Lu2O3, Sc2O3, etc.) display particular structural characteristics and physical properties. For example, Y2O3 shows up the outstanding UCL as the typical oxide host [3, 17]. The Sc2O3 has the smallest lattice parameter. The short Sc–Sc bond length in Sc2O3 can produce the short distance within an Yb3+-Er3+ pair, speeding up the Yb3+ → Er3+ energy transfer. In our previous work, Sc2O3: Er3+, Yb3+ nanostructures were obtained using a biphasic solvothermal (ST) method [17]. The red UCL in this samples are enhanced, compared with the bulk sample synthesized using a solid-state (SS) reaction. The average crystal size of nanostructures has reduced to about 200 nm, which favors the application in fluorescence imaging.
A variety of chemical techniques, including coprecipitation, solvothermal synthesis (ST), hydrothermal method (HT), sol–gel processing, thermal decomposition, etc., have been demonstrated to synthesize lanthanide-doped UC NCs [14, 18,19,20,21,22]. Optimization of synthesis procedure is critical to obtain NCs with tailored crystal size, morphology, surface functionalization, and optical properties. The HT approach is a good choice due to its convenience, exemption from pollution, and the possibility of achieving satisfying crystallinity at a relatively low temperature [23]. Zhao et al. utilized an oleic acid-mediated HT method for the synthesis of UC NaYF4 nanorods, nanotubes, and flower-patterned nano-disks [20]. Chen et al. prepared Fe3+ co-doped NaYF4: Er, Yb UC NCs by a HT method using oleic acid as a capping ligand and a surface modifier [24]. In this work, Sc2O3: Er3+, Yb3+ nanoparticles (NPs) of 19 nm in average diameters have been first synthesized through a simple oleic acid-mediated HT method. We found the stronger UCL in this Sc2O3: Er3+, Yb3+NPs samples, of which the red UCL are enhanced by a factor of 4, in comparison with that in the same optimized concentration Sc2O3 samples by ST method. The UCL enhancement can be attributed to the reduced surface groups and longer lifetimes. Additionally, the UCL property and mechanism of HT-Sc2O3: Er3+, Yb3+ NPs were investigated by the spectra distribution, power dependence, and lifetime measurement.
Experimental
Sample Preparation
The Sc2O3: Er3+, Yb3+ samples were prepared by the HT method via the hydrolysis of relevant mineral salts in an ethanol scheme. The high purity raw materials of Sc2O3, Er2O3, and Yb2O3 powers were dissolved in dilute HNO3 and deionized water to obtain cationic nitrates solutions, respectively. The Sc(NO3)3, Er(NO3)3, and Yb(NO3)3 solutions with corresponding mole ratios were dissolved in absolute ethanol (20 ml), stirring to form a homogeneous solution. Then an aqueous sodium hydroxide solution (2 ml) was added dropwise to the above mixture with stirring for 30 min, followed by adding oleic acid (1 ml), then vigorous stirring for 1 to 2 h. The resulting suspension was placed in a close Teflon-lined stainless steel autoclave with 50 ml capacity and heated at 180 °C for 24 h. After the autoclave was cooled to room temperature, naturally the precipitate was then centrifuged and washed several times with deionized water and absolute ethanol, respectively. The powder was obtained after being dried in a vacuum oven at 80 °C for 15 h and annealed 700 °C for 2 h. For comparison, we prepared Sc2O3 samples prepared by the ST method at the same sintering temperature 700 °C for 2 h [17].
Measurements and Characterization
Powder X-ray diffraction (XRD) datum was collected using Cu-Kα radiation (λ = 1.54056 Å) on an X-ray powder diffractometer (Rigaku D/Max IIA). Transmission electron microscopy (TEM) image was obtained by using a transmission electron microscope (JEM-2000EX) operating at an acceleration voltage of 200 kV. The UCL spectra were recorded with a spectrophotometer (Hitachi F-7000) and infrared spectra were performed by using a Triax 550 spectrometer (Jobin-Yvon) pumped with a power-controllable 980 nm diode laser at room temperature. Infrared spectra in transmission mode were measured on a Thermofisher Nicolet IS50 FT-IR spectrometer, using pressed KBr tablets. In fluorescence lifetime measurements, an optical parametric oscillator (OPO) was tuned to 980 nm as an excitation source, and the signals were detected by a Tektronix digital oscilloscope (TDS 3052).
Results and Discussion
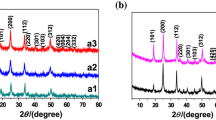
The structures characterized by the XRD patterns are shown in Fig. 1a for samples by HT method with the nominal compositions of Sc2O3: 1%Er3+, y%Yb3+ (x = 0, 5, 10, 15). The pure phase Sc2O3 was synthesized in agreement with JCPDS card 84-1884. The host lattice exhibits the mineral bixbyite structure with the \( Ia\overline{3} \) (Th2) symmetry [25]. In this structure, Sc3+ is sixfold with the effective ionic radius (0.745 Å). The Yb3+ ions owned the large ionic radius (0.868 Å) occupy Sc3+ sites to expand the lattice cell volume, making XRD peaks shift to smaller angles as Yb3+ concentration increases as shown in the magnified patterns of Fig. 1b. To further reveal the morphology and size distribution, the as-prepared Sc2O3 samples were characterized by TEM. Figure 2a shows the TEM image of HT-Sc2O3: 1%Er3+, 5%Yb3+. We obtained the sphered NPs with relatively uniform size and good monodispersity. Figure 2b depicts the histogram of the size distribution; these data were obtained from the TEM image of more than 300 NPs. The average diameter of NPs was determined to be about 19 nm.
Figure 3 shows the UCL spectra of Sc2O3: 1%Er3+, 10%Yb3+ (a) and Sc2O3: 1%Er3+, 5%Yb3+ (b) samples prepared by HT and ST methods under 980 nm excitation with an output power density of 3 mW mm−2. The strong emission bands centered at ~ 550 and 660 nm are attributed to the 4f - 4f electronic transitions of Er3+: (2H11/2, 4S3/2) → 4I15/2 and 4F9/2 → 4I15/2 transitions, respectively. The insets present the digital photographs of corresponding samples. It reveals that UCL has been dramatically enhanced for the HT sample, compared with the ST one. For HT-Sc2O3 samples, the calculated enhancement factor of red UCL is around 4, compared with corresponding ST-Sc2O3 samples. It is known that the size of samples has an influence on UCL intensity, which decreased with the decreasing of the size. However, for HT-Sc2O3 sample, it owns smaller size and more intensive UCL. It indicates the HT-Sc2O3 sample is an excellent material owned intense UCL with small size for the biological fields.
The FTIR spectra of HT-Sc2O3: 1%Er3+, 5%/10%Yb3+ and ST-Sc2O3: 1%Er3+, 5%Yb3+/10%Yb3+ samples are shown in Fig. 4. The broad band around 3429 cm− 1 is attributed to the stretching vibration of –OH in the oleic acid (OA) and water [26, 27]. The 2925 and 2850 cm− 1 absorption bands are assigned to the asymmetric and symmetric stretching vibrations of the methylene (CH2) in the long alkyl chain of the OA molecules. The sharpness of the bands indicates that the hydrocarbon chains are well ordered. The anti-symmetric methyl stretch (CH3) is seen as a shoulder on the peak at 2975 cm−1. The bands at 1200–1750 cm−1 can be assigned to the vibrations of C=O in the oleic acid molecule and CO2 in the air [28]. The transformation to carbonate might have occurred on the surface of crystallites during the heat treatment. These results evidence the existence of capping ligands on the surfaces of samples. Figure 4 shows the absorption intensities of –OH vibration for ST-Sc2O3 samples are stronger. The intensities of surface groups for HT/ST-Sc2O3: 1%Er3+, 10%Yb3+ samples are both stronger than that in co-doped 5%Yb3+ samples. The abundant surface groups with available large vibrational quanta may efficiently enhance the MPR processes, inducing the decline of luminescence.
In order to exactly describe the population mechanism in Er3+/Yb3+ co-doped HT-Sc2O3 sample, the dependence of spectral distributions on the Er3+/Yb3+ concentrations has been studied in detail.
The UCL spectra of HT-Sc2O3: x%Er3+, 10%Yb3+ (x = 0, 0.5, 1, 2) under 980 nm excitation are presented in Fig. 5a. For the fixed Yb3+ concentration at 10%, the strongest UCL is observed for Er3+ concentration around 1%. When Er3+ concentration exceeds 1%, the intensity begins to diminish because of the cross relaxation (CR) of Er3+ ions [17]. The UCL spectra of HT-Sc2O3: 1%Er3+, y%Yb3+, (y = 0, 5, 10, 15) are presented in Fig. 5b. For the Er3+ singly doped Sc2O3, its UC emission is very faint, which has been magnified 100 times. The ET process of Yb3+ → Er3+ plays a dominant role for UCL enhancement. The strongest UCL is observed for Yb3+ concentration 5% when fixed the optimal Er3+ concentration 1%.
The near infrared emission spectra in the range of 1000–1700 nm for the same variety samples are shown in Fig. 6. In the Er3+/Yb3+ co-doped samples, 980-nm photon excites Yb3+: 2F7/2 → 2F5/2 which exhibits fluorescence at 1000–1200 nm exciting Er3+ ions into 4I11/2 level through a nonresonant phonon-assisted ET process [9]. The Er3+ ions in 4I11/2 level decay nonradiatively to 4I13/2 level, then radiatively to the ground state emitting the photon around 1550 nm [9]. In Fig. 6a, as Er3+concentration increases, the Yb3+ emission has a steady decline which evidences the efficient Yb3+ → Er3+ ET. The Er3+ emission gradually increases when Er3+ concentration increases from 0 to 1%, then declines slightly as a result of the self-absorption of Er3+ ions. In Fig. 6b, Er3+: 4I13/2 emission gradually enhances when Yb3+ concentration increases from 0 to 5% but subsequently begins to decrease. As Yb3+ concentration increases, Yb3+ capacity of 980 nm photon absorption is enhanced. The Yb3+ emission intensity is shown to increase. Meantime, as the distance of Yb-Yb and Yb-Er pairs decreases, the enhanced energy migration among Yb3+ ions speeds up ET from Yb3+ to Er3+. It leads to the increased population of Er3+: 4I13/2 level but the decreased one of Yb3+: 2F5/2level. Due to the quenching of Er3+ by Yb3+ ions, the emission of Er3+: 4I13/2 → 4I15/2 reaches a maximum then drops down.
The pumping power dependences of Er3+: (2H11/2, 4S3/2) → 4I15/2 and Er3+: 4F9/2 → 4I15/2 intensities in HT-Sc2O3: 1%Er3+, 10%Yb3+ are measured under 980 nm excitation and plotted in a double logarithmic scales in Fig. 7. For the UCL processes, the UCL intensity (IUCL) depends on the pumping laser power (P) as the equation: IUCL ∝ Pn where n is the number of pumping photons absorbed per upconverted photon emitted [29]. The n value can be obtained from the slope of the linear plots between log (I) and log (P). For the two-step ET process, the n value is theoretically less than 2 due to the competition between linear decay and UC processes. Figure 7 shows the slope n values for red and green emissions are 2.5 and 2.1 in the low pump power density, respectively. It indicates, except for two-step process, that there are also the three-photon processes in HT-Sc2O3: 1%Er3+, 10%Yb3+ NPs [30, 31].
The upconversion mechanism is drawn in Fig. 8. The ET process is as follows:
-
The ET①: Yb3+: 2F5/2 + Er3+: 4I15/2 → Yb3+: 2F7/2 + Er3+: 4I11/2
-
Er3+: 4I11/2 → Er3+: 4I13/2 (MPR)
-
-
The ET②: Yb3+: 2F5/2 + Er3+: 4I13/2 → Yb3+: 2F7/2 + Er3+: 4F9/2
-
The ET③: Yb3+: 2F5/2 + Er3+: 4I11/2 → Yb3+: 2F7/2 + Er3+: 4F7/2
-
Er3+: 4F7/2 → Er3+: (2H11/2, 4S3/2) (MPR)
-
-
The ET④: Yb3+: 2F5/2 + Er3+: 4F9/2 → Yb3+: 2F7/2 + Er3+: 2H9/2
-
Er3+: 2H9/2 → Er3+: (2H11/2, 4S3/2) (MPR)
-
Er3+: (2H11/2, 4S3/2) → Er3+: 4F9/2 (MPR)
-
-
The ET⑤: Yb3+: 2F5/2 + Er3+: (2H11/2, 4S3/2) → Yb3+: 2F7/2 + Er3+: 2G7/2
To verify and make a theoretical interpretation of the UCL results mentioned above, we utilize the simplified steady-state equations.
Where σ is the absorption cross section of Yb3+ ions, I is the incident pumping power, Ni is the population density of the ith level of Yb3+, ni is the population density of ith level of Er3+ involved in the upconversion process, τi is the lifetime of ith level of Er3+and τYb is the lifetime of 2F5/2 level of Yb3+, Ci represents the ET coefficient of Yb3+ → Er3+ for steps i = 1, 2, 3, 4, 5, and W21 represents the nonradiative rate between 1 and 2 levels of the Er3+ ions.
Compared with two-step process, the UC efficiency of three-photon processes from NIR to visible is decreased [32]. Additionally, the high-photon process is prominent when pumping power is high enough. The excitations of Er3+: 4F9/2 by ET to Er3+: 2H9/2 can be neglected due to the weak pump in our experiment. By Eq. (4), the red emission intensity (IRed) can be obtained by
Due to the CR of the Er3+– Er3+ interaction is not considered, the lifetime, τ3, is a constant. That is to say, \( {\mathrm{I}}_{\mathrm{Red}}\propto {\mathrm{I}}_{\mathrm{Yb}}{\mathrm{I}}_{{\mathrm{n}}_1} \), where IYb and In1 represent the emission intensity of Yb3+: 2F5/2 and Er3+: 4I13/2, respectively. The γ3 is radiative rate of red emission. The calculated IRedvalues at various Er3+/Yb3+concentrations are presented in Fig. 9, scaled to the maximum. For comparison, the IRed values obtained directly from the UCL emission spectra are also depicted. The calculated and experimental IRed trends are consistent with each other and obtain the best value at the same Er3+/Yb3+ concentrations, demonstrating the validity of experimental data.
The three-photon green and red UC processes occurred simultaneously result in the increase of the corresponding n values. Meanwhile, the n value of red UC process increases more effectively than that of green UC process. In Fig. 8, the green and red UCL can be populated by CR, as Er3+: 4G11/2 + Er3+: 4I15/2 → Er3+: (2H11/2, 4S3/2) + Er3+: 4I13/2 and Er3+: 4G11/2 + Yb3+: 2F7/2 → Er3+: 4F9/2 + Yb3+: 2F5/2, respectively [31]. The three-photon green UCL is via a cross-relaxation process between two Er3+ ions; however, the cross-relaxation in the three-photon red UCL is between Yb3+ and Er3+ ions. Since the Yb3+ concentration is much higher than Er3+ in our experiment, the three-photon red UC process is more effective than the three-photon green UC process, resulting in a rapid increase of n value for red UCL. In addition, it should be noted that all the three-photon processes are few, so the n values deviate obviously from 3. At the high pump power density, two slopes gradually drop to 1 because UC process becomes dominant [33].
The decay curves of the Er3+: (2H11/2, 4S3/2) → 4I15/2 and 4F9/2 → 4I15/2 transitions in HT-Sc2O3 and ST-Sc2O3 samples under the 980 nm excitation wavelength have been measured and shown in Fig. 10. The decay times for red and green emissions are calculated by integrating the area under the corresponding decay curves with the normalized initial intensity. Figure 10a, b shows the green and red emission lifetimes in HT-Sc2O3: 1%Er3+, 5%Yb3+ are longer than those in ST-Sc2O3: 1%Er3+, 5%Yb3+. The lifetime is proportional to population of level. The longer values indicate the stronger red and green UCL in HT-Sc2O3 sample. In our previous report, we found our samples own the shorter decay lifetime values than that in the literature. Actually, the decay times of Er3+: (2H11/2, 4S3/2) → 4I15/2 and 4F9/2 → 4I15/2 emissions for HT/ST-Sc2O3: 1%Er3+, 5%Yb3+ samples are both close to each other. If Er3+: 4F9/2 level is populated by the MPR process from Er3+: (2H11/2, 4S3/2) levels, the decay time of Er3+: 4F9/2 level approaches to that of Er3+: 4S3/2 level. However, this MPR process is inefficient for population of Er3+: 4F9/2 level [17]. There is another non-MPR mechanism for populating the Er3+: 4F9/2level from Er3+: 4S3/2 level. The mechanism involves CR ET: Er3+: (2H11/2, 4S3/2) + Yb3+: 2F7/2 → Er3+: 4I13/2 + Yb3+: 2F5/2; then, in the same Er3+–Yb3+ pair, an energy back transfer (CRB) Yb3+: 2F5/2 + Er3+: 4I13/2 → Yb3+: 2F5/2 + Er3+: 4F9/2occurs [1]. If the CRB process dominates the main way for the population of Er3+: 4F9/2 level, the decay time of Er3+: 4F9/2 level should be almost equal to the decay time of Er3+: 4S3/2 level. The CRB process is fast and efficient at low excitation density.
Figure 11 shows the UCL spectra of three typical sesquioxides under 980 nm excitation. The Sc2O3: 1%Er3+, 5%Yb3+ sample exhibits the strongest UCL in the series of spectra. Furthermore, the emission line of Er3+: 4F9/2 level at the lowest energy side in Sc2O3 shifts to the longer wavelength side by 8 nm relative to that in Y2O3. The nearest Sc-Sc distance is 3.27 Å in Sc2O3 shorter than the Y-Y distance (3.752 Å) in Y2O3 [3, 17]. The mean Sc–O bond length (2.121 Å) in Sc2O3 is shorter than the mean Y–O bond length (2.263 Å) in Y2O3. The Er3+/Yb3+ on Sc3+ site in Sc2O3 experiences a stronger crystal field than on Y3+ site in Y2O3. The red shift of spectrum can be attributed to the large Stark splitting of Er3+ ions in Sc2O3 host. The morphologies of Y2O3 and Lu2O3 samples were also characterized by TEM as shown in the inset of Fig. 11a, b, respectively, for comparison. The obtained spherical particles are both agglomerated to bulk. The better dispersion and uniformity of Sc2O3 NPs synthesized by HT method favor its application in biological assays and medical image.
Conclusions
In summary, Sc2O3: Er3+, Yb3+ NPs about 19 nm were synthesized by a simple oleic acid-mediated HT process. The Sc2O3: Er3+, Yb3+ NPs by HT method shows the stronger UCL, of which the red UCL are enhanced by a factor of 4, in comparison with that in the same optimized concentration Sc2O3 samples by ST method. The UCL enhancement can be attributed to the reduced surface groups and longer lifetimes. The surface groups enhanced the MPR, inducing the decline of luminescence. Under the 980 nm excitation, the decay curves of Er3+: (2H11/2, 4S3/2) → 4I15/2 and 4F9/2 → 4I15/2 emissions for HT-Sc2O3: 1%Er3+, 5%Yb3+ samples are close to each other, resulting from the non-MPR mechanism for populating the Er3+: 4F9/2 level from Er3+: 4S3/2 level. The mechanism involves CR ET: Er3+: (2H11/2, 4S3/2) + Yb3+: 2F7/2 → Er3+: 4I13/2 + Yb3+: 2F5/2; then, in the same Er3+–Yb3+ pair, an energy back transfer (CRB) Yb3+: 2F5/2 + Er3+: 4I13/2 → Yb3+: 2F5/2 + Er3+: 4F9/2 occurs. Under the relatively low-power density, the slopes of the linear plots of log(I) vs log(P) for red and green emissions are 2.5 and 2.1, respectively, which are larger than 2 because of the existence of three-photon processes. Compared with the typical sesquioxides (Y2O3 and Lu2O3), the Sc2O3: 1%Er3+, 5%Yb3+ NPs exhibits the stronger UCL. Furthermore, in Sc2O3 the emission line of Er3+: 4F9/2 level at the lowest energy side shifts to the longer wavelength side by 8 nm relative to that in Y2O3 owing to the large Stark splitting of Er3+ ions in Sc2O3 host. Results show the Sc2O3: Er3+, Yb3+ nanoparticles (NPs) is an excellent material for achieving intense UCL with small size in the biological fields.
Abbreviations
- CR:
-
Cross relaxation
- ET:
-
Energy transfer
- HT:
-
Hydrothermal
- NCs:
-
Nanocrystals
- NPs:
-
Nanoparticles
- OPO:
-
Optical parametric oscillator
- ST:
-
Solvothermal
- TEM:
-
Transmission electron microscopy
- UCL:
-
Upconversion luminescence
- XRD:
-
X-ray diffraction
References
Zhang JH, Hao ZD, Li J, Zhang X, Luo YS, Pan GH (2015) Observation of efficient population of the red-emitting state from the green state by non-multiphonon relaxation in the Er3+–Yb3+ system. Light Sci Appl 4:e239
Qin W-P, Liu Z-Y, Sin C-N, Wu C-F, Qin G-S, Chen Z, Zheng K-Z (2014) Multi-ion cooperative processes in Yb3+clusters. Light Sci Appl 3:e193
Li J, Zhang JH, Hao ZD, Zhang X, Zhao JH, Luo YS (2012) Intense upconversion luminescence and origin study in Tm3+/Yb3+ codoped calcium scandate. Appl Phys Lett 101:121905
Möbert PE-A, Heumann E, Huber G, Chai BHT (1997) Green Er3+: YLiF4 upconversion laser at 551 nm with Yb3+ codoping: a novel pumping scheme. Opt Lett 22:1412–1414
Zijlmans HJMAA, Bonnet J, Burton J, Kardos K, Vail T, Niedbala RS, Tanke HJ (1999) Detection of cell and tissue surface antigens using up-converting phosphors: a new reporter technology. Anal Biochem 267:30–36
Dianoy EM (2012) Bismuth-doped optical fibers: a challenging active medium for near-IR lasers and optical amplifiers. Light Sci Appl 1:e12
Liang Y-J, Liu F, Chen Y-F, Wang X-J, Sun K-N, Pan ZW (2016) New function of the Yb3+ion as an efficient emitter of persistent luminescence in the short-wave infrared. Light Sci Appl 5:e16124
Lv RC, Yang PP, Hu B, Xu JT, Shang WT, Tian J (2017) In situ growth strategy to integrate up-conversion nanoparticles with ultrasmall CuS for photothermal theranostics. ACS Nano 11:1064–1072
Auzel F (2004) Upconversion and anti-stokes processes with f and d ions in solids. Chem Rev 104(1):139–174
Wang F, Liu XG (2009) Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem Soc Rev 38:976–989
Teng X, Zhu YH, Wei W, Wang SC, Huang JF, Naccache R, Hu WB, Tok AIY, Han Y, Zhang QC, Fan QL, Huang W, Capobianco JA, Huang L (2012) Lanthanide-doped NaxScF3+x nanocrystals: crystal structure evolution and multicolor tuning. J Am Chem Soc 134:8340–8343
Etchart I, Huignard A, Bérard M, Nordin MN, Hernández I, Curry RJ, Gillin WP, Cheetham AK (2010) Oxide phosphors for efficient light upconversion: Yb3+and Er3+ co-doped Ln2BaZnO5(Ln =Y, Gd). J Mater Chem 20:3989–3994
Dong B, Cao BS, He YY, Liu Z, Li ZP, Feng ZQ (2012) Temperature sensing and in vivo imaging by molybdenum sensitized visible Upconversion luminescence of rare-earth oxides. Adv Mater 24:1987
Paik T, Gordon TR, Prantner AM, Yun H, Murray CB (2013) Designing tripodal and triangular gadolinium oxide Nanoplates and self-assembled Nanofibrils as potential multimodal bioimaging probes. ACS Nano 7(3):2850–2859
Li T, Guo CF, Lin L (2013) Up-conversion luminescence of Er3+-Yb3+ co-doped CaIn2O4. Opt Exp 21:18281
Ruan JF, Yang ZW, Huang AJ, Zhang HL, Qiu JB, Song ZG (2018) Thermal-chromic reaction induced reversible upconversion emission modulation for switching devices and tunable upconversion emission based on defect engineering of WO3: Yb3+, Er3+ phosphor. ACS Appl Mater Interfaces 10:14941–14947
Jiu JX, An XT, Li J, Leng J, Lü W, Chen L, Wang XJ (2017) Intense red upconversion luminescence and dynamical processes in Sc2O3: Yb3+, Er3+ nanostructure. Dalton Trans 46:15954–15960
Ma YJ, Yang ZW, Zhang HL, Qiu JB, Song ZH (2018) Preparation, growth mechanism, upconversion, and near-infrared photoluminescence properties of convex-lens-like NaYF4 microcrystals doped with various rare earth ions excited at 808 nm. Cryst Growth Des 18:1758–1767
Boyer J-C, Vetrone F, Cuccia LA, Capobianco JA (2006) Synthesis of colloidal upconverting NaYF4 nanocrystals doped with Er3+, Yb3+ and Tm3+, Yb3+ via thermal decomposition of lanthanide trifluoroacetate precursors. J Am Chem Soc 128(23):7444–7445
Zhang F, Wan Y, Yu T, Zhang FQ, Shi YF, Xie SH, Li YG, Xu L, Tu B, Zhao DY (2007) Uniform nanostructured arrays of sodium rare-earth fluorides for highly efficient multicolor Upconversion luminescence. Angew Chem Int Ed 46:7976
Venkatramu V, Falcomer D, Speghini A, Bettinelli M, Jayasankar CK (2008) Synthesis and luminescence properties of Er3+-doped Lu3Ga5O12 nanocrystals. J Lumin 128:811–813
Xiang GT, Zhang JH, Hao ZD, Zhang X, Pan G-H, Chen L, Luo YS, Lü SZ, Zhao HF (2015) Solvothermal synthesis and upconversion properties of about 10 nm orthorhombic LuF3: Yb3+, Er3+ rectangular nanocrystals. J. Colloid Interface Sci 459:224–229
Li YQ, Zhang JH, Zhang X, Luo YS, Ren XG, Zhao HF, Wang XJ, Sun LD, Yan CH (2009) Near-infrared to visible upconversion in Er3+ and Yb3+ codoped Lu2O3 nanocrystals: enhanced red color upconversion and three-photon process in green color upconversion. J Phys Chem C 113:4413–4418
Tang J, Chen L, Li J, Wang Z, Zhang JH, Zhang LG, Luo YS, Wang XJ (2015) Selectively enhanced red upconversion luminescence and phase/size manipulation via Fe3+ doping in NaYF4: Yb, Er nanocrystals. Nanoscale 7:14752–14759
Lupei V, Lupei A, Gheorghe C, Ikesue A, Osiac E (2009) Energy transfer-driven infrared emission processes in rare earth-doped Sc2O3 ceramics. J Lumin 129:1862–1865
Liu JF, Li YD (2007) Synthesis and self-assembly of luminescent Ln3+-doped LaVO4 uniform nanocrystals. Adv Mater 19:1118–1122
Chen DQ, Yu YL, Huang F, Yang AP, Wang YS (2011) Lanthanide activator doped NaYb1-xGdxF4 nanocrystals with tunable down-, up-conversion luminescence and paramagnetic properties. J Mater Chem 21:6186–6192
Söderlind F, Pedersen H, Petoral RM Jr, Käll P-O, Uvdal K (2005) Synthesis and characterisation of Gd2O3 nanocrystals functionalized by organic acids. J Colloid Interface Sci 288:140–148
Pollnau M, Gamelin DR, Lüthi SR, Güdel HU, Hehlen MP (2000) Power dependence of upconversion luminescence in lanthanide and transition-metal-ion systems. Phys Rev B 61:3337
Song F, Zhang GY, Shang MR, Tan H, Yang J, Meng FZ (2001) Three-photon phenomena in the upconversion luminescence of erbium–ytterbium-codoped phosphate glass. Appl Phys Lett 79(12):1748–1750
Xiang GT, Zhang JH, Hao ZD, Zhang X, Pan G-H, Luo YS, Zhao HF (2015) Decrease in particle size and enhancement of upconversion emission through Y3+ ions doping in hexagonal NaLuF4:Yb3+/Er3+nanocrystals. Cryst Eng Comm 17:3103–3109
Suyver JF, Aebischer A, Biner D, Gerner P, Grimm J, Heer S, Krämer KW, Reinhard C, Güdel HU (2005) Novel materials doped with trivalent lanthanides and transition metal ions showing near-infrared to visible photon upconversion. Opt Mater 27:1111–1130
Suyver JF, Aebischer A, García-Revilla S, Gerner P, Güdel HU (2005) Anomalous power dependence of sensitized upconversion luminescence. Phys Rev B 71:125123
Funding
This work is supported by the National Natural Science Foundation of China (Grant No. 11504029 and 11474035), the Jilin Province Science & Technology Department (Grant No. 20170520110JH and 20170520108JH), and 2015 Programming Projects on Scientific Research of Jilin Province Department of Education.
Availability of Data and Materials
The datasets supporting the conclusions of this article are included within the article.
Author information
Authors and Affiliations
Contributions
JL and LC contributed to study design. FL and JL performed the experiments, analyzed the data, and wrote the manuscript. YXH, YRP, and JJM participated in the analyses of the results and discussion of this study. LGZ and YSL ensured UCL, FTIR, and OPO assays. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Li, F., Li, J., Chen, L. et al. Hydrothermal Synthesis and Upconversion Properties of About 19 nm Sc2O3: Er3+, Yb3+ Nanoparticles with Detailed Investigation of the Energy Transfer Mechanism. Nanoscale Res Lett 13, 372 (2018). https://doi.org/10.1186/s11671-018-2794-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-018-2794-9