Abstract
In this paper, three kinds of multi-walled carbon nanotubes (MWCNTs) with different diameters (outer diameters: 10 to 20, 30 to 50, and >50 nm) and special surface areas (200, 60, and 40 m2/g, respectively) were oxidized in commonly used liquid oxidizers: (1) concentrated nitric acid, (2) a mixture of nitric + sulfuric acids (V:V, 1:3), (3) hydrogen peroxide, (4) a mixture of hydrogen peroxide + sulfuric acid (V:V, 1:1), and (5) acidic potassium permanganate. Morphology of the pristine and oxidized MWCNTs was characterized by scanning electron microscopy which provides sufficient resolution for direct visualization of their outer diameter distribution. Full width at half maximum (FWHM) in the X-ray diffraction (XRD) investigation of the MWCNT samples before and after the oxidation process was measured. After treatment with oxidants, a clear decrease in nanotube diameters along the tube walls was observed. Decrease in the degree of crystallites started with the FWHM widening of the XRD diffraction peaks. The particle (crystallite) size (d002) calculated by Bragg's law and Scherrer equation increased depending on the kind of oxidants; the procedure can be performed using a mixture of HNO3 + H2SO4 on the surface of the MWCNTs with an outer diameter of 10 to 20nm. However, similar are the diffraction patterns of pristine and oxidized MWCNTs. Therefore, the MWCNTs that underwent oxidation process were able to preserve the first features of their structures, even though some narrowing of outer diameters and decreases in crystallites appeared. Nevertheless, the structure of MWCNTs still remains intact to be used as oxidized nanotubes in most applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Carbon nanotubes (CNTs) are divided into two types, namely single-walled CNTs (SWCNTs) and multi-walled CNTs (MWCNTs). SWCNTs are a graphite sheet rolled into a cylinder of a few micrometers in length and a few nanometers in diameter. MWCNTs consist of several such cylinders nested inside each other. MWCNTs have attracted great attention as a new kind of nanomaterial since their discovery in 1991. Carbon nanotubes have unique mechanical, electrical, magnetic, optical, and thermal properties. Owing to their inert nature, the nanotubes tend to form bundles with each other and thus do not disperse well in organic matrices in their pristine state. Suitable enhancement of the surface of MWCNTs is thus required in order to optimize their dispersion in the organic matrices. Out of various possible ways to achieve surface functionalization, chemical oxidation means of surface modification are quite common [1–4]. In this case, in typical experiments, MWCNTs with different diameters were oxidized under reflux condition in different oxidants [1–29]. The pristine and oxidized MWCNTs were then investigated with regard to their crystallites and morphologies via X-ray diffraction (XRD) and scanning electron microscopy (SEM), respectively. The objective of this research is to study the morphological and crystallographic structure of oxidized MWCNT array that will be used as the first step for other functionalization and applications.
Results and discussion
Morphology and crystallographic structure of MWCNTs
SEM
The morphology of MWCNTs before and after oxidation was characterized by SEM. The outer diameter (OD) of the carbon nanotubes varied in the three kinds of MWCNTs (10 to 20, 30 to 50, and >50 nm). The average diameter of MWCNTs before and after oxidation was measured (Table 1). Figures 1, 2, and 3 show the SEM images of MWCNTs. The nanotubes are pure and only carbon nanotubes were observed. After treatment with acids, a clear change in diameter and surface roughness along the tube walls was observed. Through the oxidation process, the diameters of MWCNTs were narrowed down gradually. Table 1 presents the erosion of nanotube surface during oxidation [1–3].
XRD
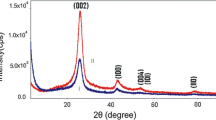
Figures 4, 5, 6, 7, 8, 9, 10, and 11 show the XRD profiles of the MWCNTs. It can be found that the pristine and oxidized samples possess a structure similar to that of graphite crystal, which indicates that the functionalization process does not change the bulk structure of the MWCNTs. The strongest and sharpest diffraction peak for all samples at around 2θ = 26.3° could be indexed as the C (002) reflection of graphite. The sharpness of the C (002) peak indicates that the graphite structure of MWCNTs was acid-oxidized without significant damage. XRD was used to measure the crystal size and interlayer spacing. Due to the CNT's intrinsic nature, the main features of the X-ray diffraction pattern of CNTs are close to those of graphite, as shown in Figures 4, 5, 6, 7, 8, 9, 10, and 11. A comparison between Figure 4 and the others shows that a graphite-like peak (0 0 2) is present at approximately 26° in 2θ. Measurements of crystal size (d) can be achieved from this peak (Tables 2 and 3) and the Scherrer equation (Equation 1). As mentioned above, the average crystallite size of the MWCNTs was determined using the XRD patterns, via the well-known Scherrer equation:
where β is the full width at half maximum (FWHM), θ is the diffraction angle, λ is the wavelength (1.54 Å), d is the particle (crystallite) size, and k is the Scherrer constant (0.91).
The Scherrer equation is derived from Bragg's law, and it is limited to nanoscale particles only. It is known that a decrease in the order of crystallinity in carbon materials will make the XRD peaks broader. Accordingly, all treated samples had a wider FWHM, which implies that the oxidation of MWCNTs had actually deteriorated the degree of crystallinity. However, amounts of it are very little (Tables 4 and 5) [2, 27].
Conclusions
Oxidation of carbon nanotubes was used as a common step in the functionalization process to increase their solubility and compatibility with different materials. However, this procedure should be used with certain caution as it can result in the destruction of the nanotube structure in the case of elevated temperatures and increased oxidation time. In this paper, the structure and morphology of pristine and oxidized MWCNTs were studied using SEM and XRD analyses. SEM examinations on MWCNTs showed that after oxidation process, the diameter of oxidized MWCNTs begin to narrow. However, comparing the SEM images of the pristine MWCNTs and that of the oxidized MWCNTs, there are practically no visual differences between them. XRD and SEM analyses revealed that the chemical treatment did not induce structural damages to the nanotubes. According to the XRD patterns, the least damaging oxidation process for creating hydrophilic sites on the hydrophobic surface of MWCNTs can be achieved using acidic potassium permanganate under reflux and mild oxidation of MWCNTs. The XRD patterns were taken to reveal detailed information about the crystallographic structure of MWCNTs. The 2θ ranged from 10° to 90°, where θ is the diffraction angle. The strongest and sharpest diffraction peak for all samples at around 2θ = 26° could be indexed as the C (0 0 2) reflection of graphite. The sharpness of the C (0 0 2) peak indicates that the graphite structure of the MWCNTs were acid-oxidized without significant damage. The crystallite size particle (d002) calculated by Bragg's law changed depending on the kind of oxidants and MWCNTs. It is known that a decrease in the order of crystallinity in carbon materials will make the XRD peaks broader. Accordingly, all treated samples have either a smaller d002 or a wider FWHM which implies that the oxidation of MWCNTs had actually deteriorated the degree of crystallinity. Moreover, the process seemed to start with widening the FWHM, followed by shifting the C (0 0 2) diffraction towards lower angles. Furthermore, this phenomenon was more significant with oxidation using the mixture HNO3 + H2SO4. These results show that there were more apparent structural changes after acid oxidation with the mixture HNO3 + H2SO4 according to the SEM images.
Methods
Material
Multi-walled carbon nanotube features are listed in Table 6.
Acid treatment of MWCNTs
Separately, three types of MWCNTs (2.0 g) with different diameters were dispersed in a 200-ml solution of 8 M HNO3 in a round-bottom flask and refluxed at 60°C for 48 h with continuous stirring (200 rpm) and ultrasonicated in an ultrasonic bath (300 W, 50 kHz) to obtain carboxyl functional groups. Upon cooling, the mixture was thoroughly washed with deionized water to remove traces of untreated acid until the pH value was 7, which signifies zero acidity. Then oxidized MWCNTs were filtered through a centrifuge and a polycarbonate filter (Whatman, pore size 0.2 μm). Then the samples were dried at 120°C in a vacuum oven for 24 h. To compare with the oxidation in HNO3, the same process was performed in a mixture of HNO3 (50 ml) + H2SO4 (150 ml) (V:V, 1:3), a mixture of H2O2 (100 ml) + H2SO4 (100 ml) and 18% H2O2 (200 ml) at 120°C, and also acidic KMnO4 (200 ml) in 95°C for 48 h. In the case of functionalization in KMnO4, the obtained solid mixture was washed with water, filtered, rewashed with concentrated HCl to remove the produced MnO2, then refiltered to remove the produced MnO2, and then refiltered again. [1–4], [11], [18], [23], [29]
Characterization methods
Scanning electron microscopy
To investigate the morphologies of MWCNTs before and after oxidation, the SEM model VEGA (TESCAN, Brno, Czech Republic) with a field emission gun was used (without gold-coated samples because the diameter of MWCNTs seems bigger with gold coating).
X-ray diffraction
XRD patterns were taken with an X-ray powder diffractometer (model GNR MPD 300, GNR Analytical Instruments Group, Novara, Italy) to reveal detailed information about the crystallographic structure of the material. The radiation used was CuKα with a wavelength of 1.54 Å. The 2θ ranged from 10° to 90°, where θ is the diffraction angle. The test condition is shown in Table 7[2, 27].
References
Ilya M, Kuznetsov VL, Simonova IA, Stadnichenko AI, Ishchenko AV, Romanenko AI, Tkachev EN, Anikeeva OB: Oxidation behavior of multiwall carbon nanotubes with different diameters and morphology. Appl Surf Sci 2012, 258: 6272–6280.
Chen J, Chen Q, Ma Q: Influence of surface functionalization via chemical oxidation on the properties of carbon nanotubes. J Colloid Interface Sci 2012, 370: 32–38.
Datsyuk V, Kalyva M, Papagelis K, Parthenios J, Tasis D, Siokou A, Kallitsis I, Galiotis C: Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46: 833–840.
Avilés F, Cauich-Rodríguez JV, Moo-Tah L, May-Pat A, Vargas-Coronado R: Evaluation of mild acid oxidation treatments for MWCNT functionalization. Carbon 2009, 47: 2970–2975.
Dandekar A, Baker RTK, Vannice MA: Characterization of activated carbon, graphitized carbon fibers and synthetic diamond powder using TPD and DRIFTS. Carbon 1998, 36: 1821–1831.
Pereira CMC, Novoa P, Martins M, Forero S, Hepp F, Pambaguian L: Characterization of carbon nanotube 3D-structures infused with low viscosity epoxy resin system. Compos Struct 2010, 92: 2252–2257.
Tsang SC, Chen YK, Harris PJF, Green MLH: A simple chemical method of opening and filling carbon nanotubes. Nature 1994,372(6502):59–62.
Moreno-Castilla C, Carrasco-Marin F, Maldonado-Hodar FJ, Rivera-Utrilla J: Effects of non-oxidant and oxidant acid treatments on the surface properties of an activated carbon with very low ash content. Carbon 1998, 36: 145–151.
Zhang J, Zou H, Qing Q, Yang Y, Li Q, Liu Z, Gou X, Du Z: Effect of chemical oxidation on the structure of single-walled carbon nanotubes. J Phys Chem 2003, B107: 3712–3718.
Aksoylu AE, Madalena M, Freitas A, Fernando M, Pereira R, Figueiredo JL: The effects of different activated carbon supports and support modifications on the properties of Pt/AC catalysts. Carbon 2001, 39: 175–185.
Esumi K, Ishigami M, Nakajima A, Sawada K, Honda H: Chemical treatment of carbon nanotubes. Carbon 1995,34(2):279–281.
Figueiredo JL, Pereira MFR, Freitas MMA, Orfao JJM: Characterization of active sites on carbon catalysts. Ind Eng Chem Res 2007, 46: 4110–4115.
Chen J, Hamon MA, Hu H, Chen Y, Rao AM, Eklund PC, Haddon RC: Solution properties of single-walled carbon nanotubes. Science 1998,282(5386):95–98.
Cuervo MR, Asedegbega-Nieto E, Díaz E, Ordóñez S, Vega A, BelénDongil A, Rodríguez-Ramos I: Modification of the adsorption properties of high surface area graphites by oxygen functional groups. Carbon 2008, 46: 2096–2106.
Zhao B, Hu H, Haddon RC: Synthesis and properties of a water-soluble single-walled carbon nanotube-poly(maminobenzene sulfonic acid) graft copolymer. Adv Funct Mater 2004,14(1):71–76.
Osorio AG, Silveira ICL, Bueno VL, Bergmann CP: H 2 SO 4 /HNO 3 /HCl—functionalization and its effect on dispersion of carbon nanotubes. Appl Surf Sci 2008, 255: 2485–2489.
Lin ST, Wei KL, Lee TM, Chiou KC, Lin JJ: Functionalizing multi-walled carbon nanotubes with poly(oxyalkylene)- amidoamines. Nanotechnology 2006,17(13):3197–3203.
Liang Y, Zhang H, Yi B, Zhang Z, Tan Z: Preparation and characterization of multi-walled carbon nanotubes supported PtRu catalysts for proton exchange membrane fuel cells. Carbon 2005, 43: 3144–3152.
Nobili MD, Bragato G, Alcaniz JM, Puigbo A, Comellas L: Characterization of electrophoretic fractions of humic substances with different electrofocusing behaviour. Soil Sci 1990,150(5):763–770.
Ötvös Z, Onyestyák G, Hance A, Kiricsi I, Rees LVC: Surface oxygen complexes as governors of neopentane sorption in multiwalled carbon nanotubes. Carbon 2006, 44: 1665–1672.
Figueiredo JL, Pereira MFR, Freitas MMA, Órfão JJM: Modification of the surface chemistry of activated carbons. Carbon 1999, 37: 1379–1389.
Tran MQ, Tridech C, Alfrey A, Bismarck A, Shaffer MSP: Thermal oxidative cutting of multi-walled carbon nanotubes. Carbon 2007,45(12):2341–2350.
Toebes ML, van Heeswijk JMP, Bitter JH, van Dillen AJ, de Jong KP: The influence of oxidation on the texture and the number of oxygen-containing surface groups of carbon nanofibers. Carbon 2004, 42: 307–315.
Ajayan PM, Ebbesen TW, Ichihashi T, Iijima S, Tanigaki K, Hiura H: Opening carbon nanotubes with oxygen and implications for filling. Nature 1993,362(6420):522–525.
Barroso-Bujans F, Fierro JLG, Rojas S, Sánchez-Cortes S, Arroyo M, López-Manchado MA: Degree of functionalization of carbon nanofibers with benzenesulfonic groups in an acid medium. Carbon 2007, 45: 1669–1678.
Hu H, Zhao B, Itkis ME, Haddon RC: Nitric acid purification of single-walled carbon nanotubes. J PhysChem B 2003,107(50):13838–13842.
Yu-Chun C, Wei-Hsiang L, Yung-Chia C: The influence of treatment duration on multi-walled carbon nanotubes functionalized by H 2 SO 4 /HNO 3 oxidation. Applied Surface Science 2011, 257: 2401–2410.
Wang SG, Liu XW, Gong WX, Nie W, Gao BY, Yue QY: Adsorption of fulvic acids from aqueous solutions by carbon nanotubes. J Chem Technol Biotechnol 2007,82(8):698–704.
Li M, Boggs M, Beebe TP, Huang CP: Oxidation of single-walled carbon nanotubes in dilute aqueous solutions by ozone as affected by ultrasound. Carbon 2008, 46: 466–475.
Acknowledgments
The authors wish to acknowledge Professor P. Davami (Razi Metallurgical Research Center, Tehran, Iran) for providing technical assistance provided and also for conducting the SEM, XRD, FT-IR, TGA, and titration equipment and experiment.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
Both authors declare that they have no competing interests.
Authors’ contributions
H KH carried out the result analysis, participated to draft the manuscript and in the manuscript elaboration, carried out the experiments, and obtained most of the experimental images. O M coordinated the project, discussed the results, and helped to draft the manuscript. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Khani, H., Moradi, O. Influence of surface oxidation on the morphological and crystallographic structure of multi-walled carbon nanotubes via different oxidants. J Nanostruct Chem 3, 73 (2013). https://doi.org/10.1186/2193-8865-3-73
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-73