Abstract
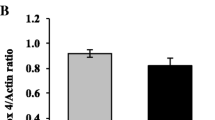
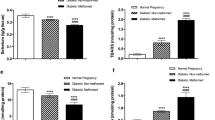
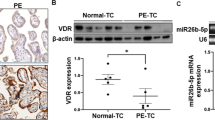
Matrix metalloproteinases (MMPs) are proteolytic enzymes related to a proinflammatory environment in several diseases, including diabetes, which can be activated by reactive nitrogen species. This work aimed to determine MMP-2 and MMP-9 activities and nitration in term placentas from type 2 diabetic patients and verify the hypothesis that peroxynitrites are positive regulators of placental MMP-2 and MMP-9 activities. For this purpose, term placentas from healthy and type 2 diabetic patients were analyzed for MMP-2 and MMP-9 levels and activities, protein nitration, and nitration of MMP-2 and MMP-9. Villous explants were cultured in the presence of peroxynitrites for further evaluation of MMP-2 and MMP-9 activities. We found that MMP-2 and MMP-9 activities were increased in term placentas from diabetic patients. These changes were found even when MMP-2 protein concentrations were diminished and MMP-9 protein concentrations were not changed in the diabetic group. Increased protein nitration and specific nitration of MMP-2 and MMP-9 were found in term placentas from diabetic patients. Peroxynitrites were able to increase the activity of placental MMP-2 and MMP-9. Taken together, this study has shown for first time that peroxynitrites can nitrate and activate MMP-2 and MMP-9 in the placenta, a nitrative pathway possibly related to MMPs overactivity in the placentas from type 2 diabetic patients.
Similar content being viewed by others
References
Balsells M, Garcia-Patterson A, Gich I, Corcoy R. Maternal and fetal outcome in women with type 2 versus type 1 diabetes melli-tus: a systematic review and metaanalysis. J Clin Endocrinol Metab. 2009;94(11):4284–4291.
Michael Weindling A. Offspring of diabetic pregnancy: short-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):111–118.
Melamed N, Hod M. Perinatal mortality in pregestational diabetes. Int J Gynaecol Obstet. 2009;104(suppl 1):S20–S24.
Plagemann A, Harder T, Dudenhausen JW. The diabetic pregnancy, macrosomia, and perinatal nutritional programming. Nestle Nutr Workshop Ser Pediatr Program. 2008;61:91–102.
Thornburg KL, O'Tierney P, Louey S. The placenta is a programming agent in cardiovascular disease. Placenta. 2010;31(suppl): 54–59.
Jansson T, Myatt L, Powell TL. The role of trophoblast nutrient and ion transporters in the development of pregnancy complications and adult disease. Curr Vase Pharmacol. 2009;7(4): 521–533.
Omu AE, Al-Azemi MK, Omu FE, et al. Butyrylcholinesterase activity in women with diabetes mellitus in pregnancy: correlation with antioxidant activity. J Obstet Gynaecol. 2010;30(2):122–126.
Pacher P, Obrosova IG, Mabley JG, Szabo C. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem. 2005;12(3):267–275.
Lyall F, Gibson JL, Greer IA, Brockman DE, Eis AL, Myatt L. Increased nitrotyrosine in the diabetic placenta: evidence for oxidative stress. Diabetes Care. 1998;21(10): 1753–1758.
Horvath EM, Magenheim R, Kugler E, et al. Nitrative stress and poly(ADP-ribose) polymerase activation in healthy and gestational diabetic pregnancies. Diabetologia. 2009;52(9): 1935–1943.
San Martin R, Sobrevia L. Gestational diabetes and the adenosine/ L-arginine/nitric oxide (ALANO) pathway in human umbilical vein endothelium. Placenta. 2006;27(1):1–10.
Lappas M, Hiden U, Froehlich J, Desoye G, Haugel-De Mouzon S, Jawerbaum A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid Redox Signal. 2011; 15(12):3061–3100.
Pustovrh MC, Jawerbaum A, Capobianco E, et al. Oxidative stress promotes the increase of matrix metalloproteinases-2 and -9 activities in the feto-placental unit of diabetic rats. Free Radic Res. 2005;39(12):1285–1293.
Pustovrh C, Jawerbaum A, Sinner D, et al. Membrane-type matrix metalloproteinase-9 activity in placental tissue from patients with pre-existing and gestational diabetes mellitus. Reprod Fertil Dev. 2000;12(5–6):269–275.
Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14(17): 2123–2133.
Huang SC, Sheu BC, Chang WC, Cheng CY, Wang PH, Lin S. Extracellular matrix proteases-cytokine regulation role in cancer and pregnancy. Front Biosci. 2009;14:1571–1588.
Cockle JV, Gopichandran N, Walker JJ, Levene MI, Orsi NM. Matrix metalloproteinases and their tissue inhibitors in preterm perinatal complications. Reprod Sci. 2007;14(7): 629–645.
Cohen M, Bischof P. Factors regulating trophoblast invasion. Gynecol Obstet Invest. 2007;64(3):126–130.
Valdes G, Corthorn J. Review: the angiogenic and vasodilatory utero-placental network. Placenta. 2011;32(suppl 2):S170–S175.
Wang W, Sawicki G, Schulz R. Peroxynitrite-induced myocardial injury is mediated through matrix metalloproteinase-2. Cardio-vasc Res. 2002;53(1):165–174.
Gu Z, Kaul M, Yan B, et al. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297(5584): 1186–1190.
Harris LK, Mccormick J, Cartwright JE, Whitley GS, Dash PR. S-nitrosylation of proteins at the leading edge of migrating tropho-blasts by inducible nitric oxide synthase promotes trophoblast invasion. Exp Cell Res. 2008;314(8):1765–1776.
Mandal M, Mandal A, Das S, Chakraborti T, Sajal C. Clinical implications of matrix metalloproteinases. Mol Cell Biochem. 2003;252(l–2):305–329.
Derosa G, D'angelo A, Tinelli C, et al. Evaluation of metallopro-teinase 2 and 9 levels and their inhibitors in diabetic and healthy subjects. Diabetes Metab. 2007;33(2):129–134.
Thrailkill KM, Bunn RC, Moreau CS, et al. Matrix metalloproteinase-2 dysregulation in type 1 diabetes. Diabetes Care. 2007;30(9):2321–2326.
Pustovrh MC, Jawerbaum A, Capobianco E, White V, Lopez-Costa JJ, Gonzalez E. Increased matrix metalloproteinases 2 and 9 in placenta of diabetic rats at midgestation. Placenta. 2005; 26(4):339–348.
Pustovrh C, Jawerbaum A, Sinner D, White V, Capobianco E, Gonzalez E. Metalloproteinase 2 activity and modulation in uterus from neonatal streptozotocin-induced diabetic rats during embryo implantation. Reprod Fertil Dev. 2002;14(7–8): 479–485.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254.
White V, Capobianco E, Higa R, et al. Increased nitration and diminished activity of copper/zinc superoxide dismutase in placentas from diabetic rats. Free Radic Res. 2010;44(12):1407–1415.
Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26(8):587–596.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2): 351–358.
Szabo C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol Lett. 2003;140–141:105–112.
Pustovrh MC, Jawerbaum A, White V, et al. The role of nitric oxide on matrix metalloproteinase 2 (MMP2) and MMP9 in placenta and fetus from diabetic rats. Reproduction (Cambridge, England). 2007;134(4):605–613.
Macmillan-Crow LA, Thompson JA. Immunoprecipitation of nitrotyrosine-containing proteins. Methods Enzymol. 1999;301: 135–145.
Jawerbaum A, White V. Animal models in diabetes and pregnancy. Endocr Rev. 2010;31(5):680–701.
Jawerbaum A, Gonzalez E. Diabetic pregnancies: the challenge of developing in a pro-inflammatory environment. Curr Med Chem. 2006;13(18):2127–2138.
Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16(5):558–564.
Dean RA, Overall CM. Proteomics discovery of metalloproteinase substrates in the cellular context by iTRAQ labeling reveals a diverse MMP-2 substrate degradome. Mol Cell Proteomics. 2007;6(4):611–623.
Demir-Weusten AY, Seval Y, Kaufmann P, Demir R, Yucel G, Huppertz B. Matrix metalloproteinases-2, -3 and -9 in human term placenta. Acta Histochem. 2007;109(5):403–412.
Weiss A, Goldman S, Shalev E. The matrix metalloproteinases (MMPS) in the decidua and fetal membranes. Front Biosci. 2007;12:649–659.
Schulz R. Intracellular targets of matrix metalloproteinase-2 in cardiac disease: rationale and therapeutic approaches. Annu Rev Pharmacol Toxicol. 2007;47:211–242.
Freise C, Erben U, Muche M, et al. The alpha 2 chain of collagen type VI sequesters latent proforms of matrix-metalloproteinases and modulates their activation and activity. Matrix Biol. 2009; 28(8):480–489.
Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002; 115(pt 9):3719–3727.
Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med. 2004;37(6):768–784.
Hu J, Van Den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6(6):480–498.
Manicone AM, Mcguire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2008;19(1):34–41.
Webster RP, Roberts VH, Myatt L. Protein nitration in placenta-functional significance. Placenta. 2008;29(12):985–994.
Webster RP, Brockman D, Myatt L. Nitration of p38 MAPK in the placenta: association of nitration with reduced catalytic activity of p38 MAPK in pre-eclampsia. Mol Hum Reprod. 2006; 12(ll):677–685.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Capobianco, E., White, V., Sosa, M. et al. Regulation of Matrix Metalloproteinases 2 and 9 Activities by Peroxynitrites in Term Placentas From Type 2 Diabetic Patients. Reprod. Sci. 19, 814–822 (2012). https://doi.org/10.1177/1933719111434544
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719111434544