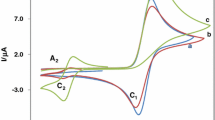
The electrochemical oxidation of catechol (1) in the presence of sulfanilic acid (2) was investigated. Some electrochemical (EC) techniques such as cyclic voltammetry and controlled-potential coulometry were used. The oxidation reaction of catechol (1) with periodate in the presence of sulfanilic acid (2) was also investigated spectrophotometrically. The results indicate that the o-quinone derived from catechol participate in Michael addition reaction with sulfanilic acid (2). In addition, according to the ECE mechanism, the observed homogeneous rate constant (kobs) for the reaction of o-quinone derived from catechol (1) with sulfanilic acid (2) has been estimated by digital simulation of cyclic voltammograms.
Similar content being viewed by others
Rights and permissions
About this article
Cite this article
Nematollahi, D., Afkhami, A., Mosaed, F. et al. Investigation of the electro-oxidation and oxidation of catechol in the presence of sulfanilic acid. Res Chem Intermediat 30, 299–309 (2004). https://doi.org/10.1163/156856704323034030
Published:
Issue Date:
DOI: https://doi.org/10.1163/156856704323034030