Abstract
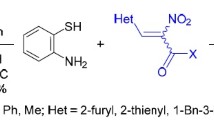
(±)cis-2-(4-methoxyphenyl)-3-hydroxy/methoxy-6-methoxy/8-methoxy-2,3-dihydro-1,5-benzothiazepin-4-[5H/5-chloroacetyl/5-(4′-methylpiperazino-1′)acetyl]-ones and nucleosides viz; (±)cis-2-(4-methoxyphenyl)-3,6-dimethoxy-2,3-dihydro-1,5-benzothiazepin-4-[5-(2,3,5-tri-O-benzoyl-β-D-ribofuranosyl)]-one and (±)cis-2-(4-methoxyphenyl)-3,8-dimethoxy-2,3-dihydro-1,5-benzothiazepin-4-[5-(2,3,5-tri-O-benzoyl-β-D-ribofuranosyl)]-one have been synthesized by the condensation of substituted-2-aminobenzenethiols with methyl (±)trans-3-(4-methoxyphenyl)glycidate in xylene and by stirring the 3-methoxy derivative of 1,5-benzothiazepin-4(5H)-ones with sugar namely β-D-ribofuranosyl-1-acetate-2,3,5-tribenzoate at 155–160 °C in vacuo for 10 hours, respectively.
The synthesized compounds have been characterized by the elemental analyses and spectral data and screened for their antimicrobial activity.
Similar content being viewed by others
References
E. Fischer and B. Helferich, Ber. Dtsch. Chem. Ges. 47, 210 (1914).
I. Verheggen, A.V. Aerschot, S. Toppet, R. Snoeck, G. Janssen, J. Balzarini, E. de Clereq, and P. Herdewijn, J. Med. Chem. 3, 2033 (1993).
I. Verheggen, A.V. Aerschot, L.V. Meervelt, J. Rozenski, L. Wiebe, R. Snoeck, G. Andrei, J. Balzarini, P. Claes, E. De Clereq, and P. Herdewijn, J. Med. Chem. 38, 826 (1995).
V. Girijavallabhan, K. Ganguly, A.B. Cooper, R. Lovey, D. Loebenberg, D. Rane, J. Desai, R. Pike, and E. Jao. In: P.H. Bently and R. Ponsford (Eds.), Recent Advances in the Chemistry of Anti Infective Agents, The Royal Society of Chemistry, Cambridge, 1993, 192pp.
L. Agrofoglio, E. Suhas, A. Farese, R. Condom, S.R. Challand, R.A. Earl, and R. Guedj, Tetrahedron 50, 10611 (1994).
M.J. Mulvihill and M.J. Miller, Tetrahedron 54, 6605 (1998).
B. Doboszewski and P. Herdewijn, Tetrahedron 52, 165 (1996).
J. Balzarini, G.J. Kang, M. Dalal, P. Herdewijn, E. De Clercq, S. Broder, and D.G. Johns, Mol. Pharmacol 32, 162 (1987).
A.M. Attia, G.H. Elgemeie, and L.A. Shahada, Tetrahedron 53(51), 17441 (1997).
A. Odawara, Y. Sasaki, S. Murata, and H. Narita (Tanabe Seiyaku, Co., Ltd.) Eur. Pat. Appl. Ep 476, 854 (Cl. A 61K31/62), 25 Mar, 1992, JP Appl. 90/243, 728, 17 Sep 1990; 6 pp.; Chem. Abstr. 117, 11427 (1992).
I.R. Buxton, A. Brown, H. Gitchlly, Leslic, T. Stewart, S. Malkowska, A. Therese, D.A. Prater, R.B. Miller (Euro Eoltique SA) Eur. Pat. Appl. EP 527, 638 (Cl. A61 K31/55), 17 Feb. 1993, G.B. Appl. EP 527, 638; Chem. Abstr. 118, 240944 (1993).
De. Hendrickson, D.C. Dimitt, M.S. Williams, P.F. Skultely, M.J. Batezor (Marion Mersell Dow Inc.) Eur Pat. Appl. EP 514, 814 (Cl. A61 K31/55), 25 Nov. 1992, 25 Appl. 702, 567, 20 May 1991; 24 pp., Chem. Abstr. 118, 66884 (1993).
Z.P. Harovitz, Psychosomatics 6, 281 (1965).
Z.P. Nygren, R. Larsson, and L. Slater, J. Cell Pharmacol. 2(4), 202 (1991).
K. Kikkawa, S. Murata, H. Iwasaki, W. Toriuni, K. Banno, and T. Naga, Arzneim Forsch. 42(6), 781 (1992).
A. Soriabine, J. Cardiovasc. Pharmacol. 9, 53 (1987).
Harari, Yakuhin Kogyo Co. Ltd. Jpn. Kokai Tokkyo, Koho JP 58, 113, 186 (83, 113, 186) (Cl. CO7D279/16), 05 Jul. 1983, Appl. 81/210, 918 28 Dec. 1981; 6 pp.; Chem. Abstr. 99, 158489 (1983).
A. Ooishi, M. Takeda, H. Nakajima, H. Nagao, Tanabe Seiyavu Co. Ltd., Jpn, Kokai Tokkyo Koho Jp 61, 267, 520 (86, 267, 520) (Cl-A61K31/55), 27 Nov. 1986, JP Appl. 84/253, 820, 29 Nov. 1984; 11 pp.; Chem. Abstr. 106, 149478 (1987).
S. Murakami, T. Ikebe, I. Hakamada, O. Yaoka, Yoshitomi Pharmaceuticals Industries Ltd., Eur. Pat. Appl. EP 292, 923 (Cl. CO7K5/06), 30 Nov. 1987, JP Appl. 87/129, 414, 25 May 1987; 21 pp.; Chem. Abstr. 110, 212635 (1989).
H. Iwasaki, K. Kitamura, and A. Ohtani, Tanabe Seiyaku Co. Ltd., Austrian AT 390,880 (Cl.A61K31/55), 10 Jul. 1990 Appl. 8712, 627, 18 Oct. 1987; 6 pp., Chem. Abstr. 115, 22228 (1991).
N.K. Ahmed, Marion Merrel Dow, Inc., Eur. Pat. Appl. EP 430, 036 (Cl.A61K31/55), 05 Jun 1991, US App. 440, 121, 22 Nov. 1989; 7 pp.; Chem. Abstr. 116, 717 (1992).
A.W. Bauer, W.M.M. Kibby, J.C. Sherris, and M. Turk, Ann. J. Clin. Path 45, 493 (1966).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Singh, G., Sharma, A.K., Kumar, N. et al. A step forward in the field of drug design: 1,5-benzothiazepines and their nucleosides. Res Chem Intermed 26, 445–453 (2000). https://doi.org/10.1163/156856700X00444
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856700X00444