Abstract
Cerium enhances the durability of polymer electrolyte membrane (PEM) fuel cells by scavenging reactive radical species which are generated during operation. However, during cell fabrication, conditioning, discharge, and during fuel cell operation, Ce dissolves and is transported within the membrane-electrode-assembly (MEA) due to gradients in ionic potential (migration),1–3 ionic concentration (diffusivity),1,4 and ionomer hydration (convection).5,6 Ce migration is detrimental because (i) its accumulation in the CL ionomer can diminish the electrode's proton conductivity, which results in sub-optimal performance, especially at high current density7,8; and (ii) its depletion may leave an ionomer region more susceptible to radical attack. Therefore, it is necessary to quantify these transport mechanisms under a range of operating conditions in order to understand their effects on cell performance/durability and determine the necessity of further Ce stabilization.
To understand the effects of potential gradients and relative humidity (RH) on Ce migration, ex situ experiments were performed using Nafion™ NR-211 (Ion Power, Inc.) which was doped in-house using Ce(III) nitrate to ~5% ion-exchanged Ce. PEM specimens were operated in 4-electrode H2 pump mode in a standard conductivity cell (Scribner Associates) under a range of temperatures and RHs. The evolution of Ce profiles as a function of charge transfer was quantified using X-ray fluorescence, which were fit using our previously-developed transient, 1-D transport model in order to decouple the competing effects of migration and diffusion.1 The model was updated to capture convection due to hydration gradients, as well as variations in λ (defined as nH2O/nSO3-) and conductivity as a function of Ce exchange fraction. As shown in Figure 1a, diffusion experiments performed in the absence of potential gradients showed excellent agreement with simultaneous 2-parameter migration and diffusivity fitting of a single migration experiment, validating the efficiency and effectiveness of this technique. Figure 1 shows modeled values for (a) diffusivity and (b) migration over a range of temperatures and λs.
Convection experiments were also conducted using a differential RH cell, adapted from the GM/Giner design.9 Since no potential gradient is present here, the convection coefficients were fit by employing the isothermal diffusivity-λ relations determined from the H2 pump experiments. Results of all three transport coefficients will be presented over a range of relevant operating conditions and 2-D cell model results demonstrating the impact on cell performance will be discussed. The findings of these studies may also be analogously applied to Co and Fe cations, which dissolve from Pt-Co electrocatalysts and system balance of plant components, respectively, and are expected show transport characteristics in PFSA.
Acknowledgement: This research is supported by the U.S. Department of Energy Fuel Cell Technologies Office, through the Fuel Cell Performance and Durability (FC-PAD) Consortium (Fuel Cells Program Manager: Dimitrios Papageorgopoulos and Technical Development Manager: Greg Kleen).
References
A. M. Baker, S. K. Babu, R. Mukundan, S. G. Advani, A. K. Prasad, D. Spernjak, and R. L. Borup, J. Electrochem. Soc., 164, 1272–1278 (2017).
A. M. Baker, D. Torraco, E. J. Judge, D. Spernjak, R. Mukundan, R. L. Borup, S. G. Advani, and A. K. Prasad, ECS Trans., 69, 1009–1015 (2015).
Y. Cai, J. M. Ziegelbauer, A. M. Baker, W. Gu, R. S. Kukreja, A. Kongkanand, M. F. Mathias, R. Mukundan, and R. L. Borup, J. Electrochem. Soc., 165, F3132–F3138 (2018).
F. D. Coms and A. B. McQuarters, ECS Trans., 86, 395–405 (2018).
A. M. Baker, R. Mukundan, D. Spernjak, E. J. Judge, S. G. Advani, A. K. Prasad, and R. L. Borup, J. Electrochem. Soc., 163, F1023–F1031 (2016).
Y.-H. Lai, K. M. Rahmoeller, J. H. Hurst, R. S. Kukreja, M. Atwan, A. J. Maslyn, and C. S. Gittleman, J. Electrochem. Soc., 165, F3217–F3229 (2018).
D. Banham, S. Y. Ye, T. Cheng, S. Knights, S. M. Stewart, M. Wilson, and F. Garzon, J. Electrochem. Soc., 161, F1075–F1080 (2014).
E. L. Redmond, S. M. Wriston, and J. L. Szarka III, ECS Trans., 80, 633–641 (2017).
S. Kumaraguru, "Durable High Power Membrane Electrode Assembly with Low Pt Loading," Fuel Cell R&D Annual Merit Review Proceedings, (2018).
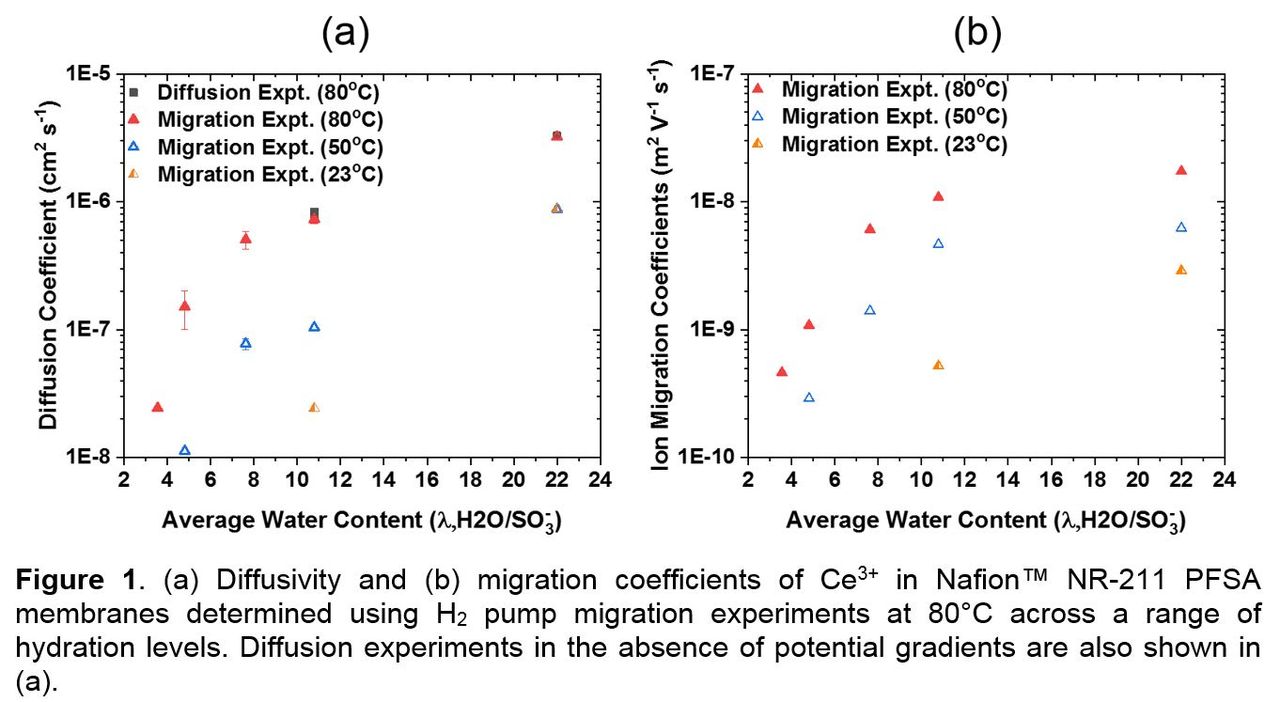
Figure 1
