Abstract
Cu-based electrode materials for the CO2 reduction reaction (CO2RR) are of special interest in current research, as Cu is the only elemental metal that catalyzes the electrochemical reduction of CO2 towards short-chain hydrocarbons [1]. This provides the possibility of converting the greenhouse gas CO2 into e-fuels or base chemicals while simultaneously acting as storage for fluctuating renewable energy sources. However, tuning the selectivity in CO2RR towards desired products is still a challenging task. Not only are the reaction mechanisms not entirely understood, but also the complex interactions of local conditions in electrode proximity, i.e. local pH, buffer capacity or cation effects, are also subject of ongoing discussions [2].
The common use of diluted KHCO3 electrolyte in CO2RR demonstrates how this interplay can be employed to one's advantage: Low buffer capacity leads to high pH in electrode proximity, which in turn favors C2+ product reaction pathways over undesired hydrogen formation [3]. Moreover, potassium as counterion has a more favorable effect on pH buffering than smaller cations and has been proven to accelerate CO2 activation [2].
For the investigation of local pH in electrochemical cells several in operando methods have been developed, e.g. optical methods like infrared or Raman spectroscopy or scanning probe microscopy techniques [4]. In our previous work, we presented a new method for spectroelectrochemical pH determination by in operando13C Nuclear Magnetic Resonance (NMR), where we operated an electrochemical cell for CO2RR in a standard 5 mm NMR tube and evaluated the local pH using the CO2(aq)/HCO3-/CO32- equilibrium [5].
The present work expands this method with magnetic resonance imaging (MRI) techniques to spatially resolve local pH measurements. The working electrode was placed perpendicular to magnetic field gradients used for MRI (cf. Figure 1a) to obtain NMR spectra as a function of distance to electrode. Phase-encoded chemical shift imaging using spin echoes was applied to subdivide the sensitive volume into slices, each of which contributes a single spectrum.
The results of 13C chemical shift imaging of 0.5 M KHCO3 electrolyte are depicted in Figure 1b and 1c. The chemical shift of the coalesced HCO3-/CO32- resonance serves as pH sensor, as it is highly pH dependent [6]. Increasing chemical shifts near the electrode were assigned to an increase in pH value from 7.2 at the beginning up to a pH of ca. 9 after 3 h of electrolysis at constant current of 2.1 mA/cm².
The CO2 concentration decreased below the detection limit shortly after start of the experiment due to the pH shift. Still, typical CO2RR products were detected by ex situ1H NMR using water suppression, confirming that CO2 was reduced from equilibrium with HCO3- [7].
When investigating NaHCO3 as electrolyte in a broadband NMR probe, the 23Na and 13C spectra could be acquired alternatingly. Using this method, local pH profiles can be directly correlated with concentration profiles of dissolved species such as CO2(aq) or the HCO3-/CO32- anions as well as Na+ cations.
In conclusion, this study shows the evolution of pH profiles over time and how this local pH effect depends on the applied potential. Furthermore, this interaction will be put into relation to cation concentration profiles as well as ex situ product analysis to give new insights into the electrolyte chemistry of Cu-catalyzed CO2RR.
References
[1] Y. Hori, Electrochemical CO2 Reduction on Metal Electrodes, in Modern Aspects of Electrochemistry, edited by C. G. Vayenas, R. E. White, M. E. Gamboa-Aldeco (Springer-Verlag, s.l., 2008), 89
[2] B. Deng, M. Huang, X. Zhao, S. Mou, F. Dong, ACS Catal. 12, 331 (2022)
[3] T. Burdyny, W. A. Smith, Energy Environ. Sci. 12, 1442 (2019)
[4] M. C. Monteiro, M. T. Koper, Current Opinion in Electrochemistry 25, 100649 (2021)
[5] M. Schatz, S. Jovanovic, R.-A. Eichel, J. Granwehr, Sci Rep 12 (2022)
[6] S. Moret, P. J. Dyson, G. Laurenczy, Dalton Trans. 42, 4353 (2013)
[7] M. Dunwell, Q. Lu, J. M. Heyes, J. Rosen, J. G. Chen, Y. Yan, F. Jiao, B. Xu, J. Am. Chem. Soc. 139, 3774 (2017)
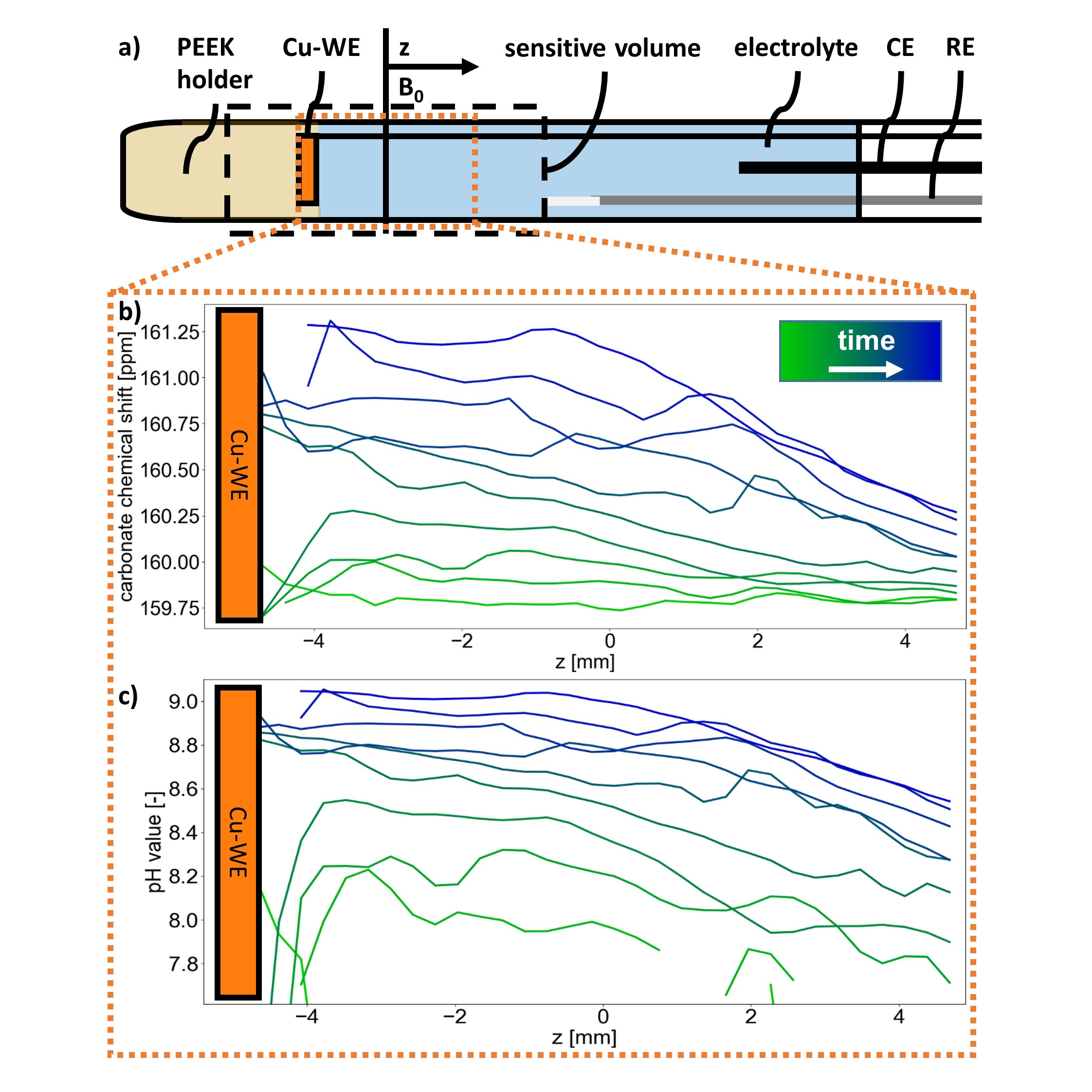
Figure 1

