Abstract
This article discusses the options and challenges of dynamic models for the diagnosis and operation of Li-ion batteries. It provides a concise yet understandable overview on models and dynamics, and it discusses future developments needed to progress the field. The diagnosis and operation of batteries require an understanding of the main processes and their dynamics, parameters, and time constants. Processes with large time constants, such as thermal transport are equally important for safe high-performance operation as are processes with shorter time constants such as diffusion. Depending on the specific problem or operating condition, taking all of the scales into account is often unavoidable. Three separate, yet closely connected model classes are reviewed in terms of physical insight and their capabilities and limits: mechanistic models, equivalent circuit models, and data-driven models. We provide guidance for the selection of a suitable model for the particular diagnosis and operation problem of interest. The optimization of battery diagnosis and operation require versatile and simple models that span multiple time scales and allow physical insight and ease of parameterization. Fusing the existing modeling approaches may help to fully exploit their potential while integrating first-principles physical insight and measurement data.
Export citation and abstract BibTeX RIS
Li-ion batteries power portable equipment and appliances, are essential components in electrical cars, and bear large potential as buffer and storage elements in electrical grids to overcome fluctuations caused by the intermittent nature of renewable energy sources. For many applications, such as in automotives or energy storage, Li-ion batteries need to operate at the upper performance limits to provide cost-effective solutions. Operating outside these limits causes fast deterioration and may lead to uncontrollable behavior.1,2 As such, optimal design and diagnosis of cell state and optimal operation need to be addressed carefully at all levels, from cell to module and system. Dynamic mathematical models provide a means to address these challenges.
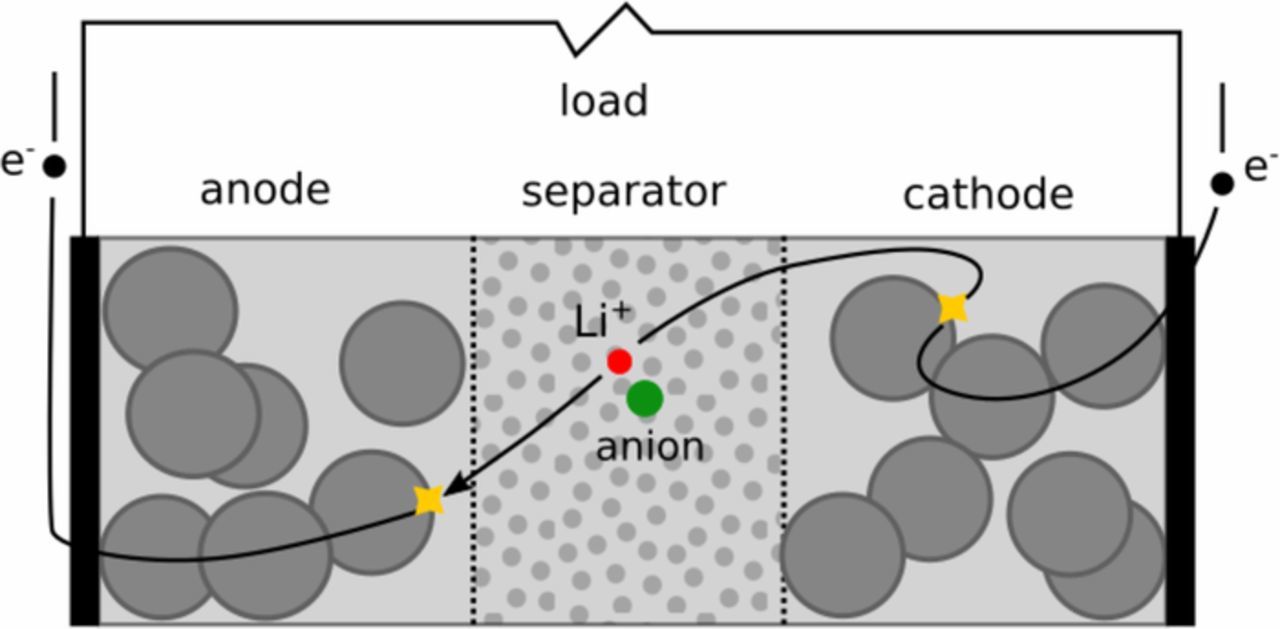
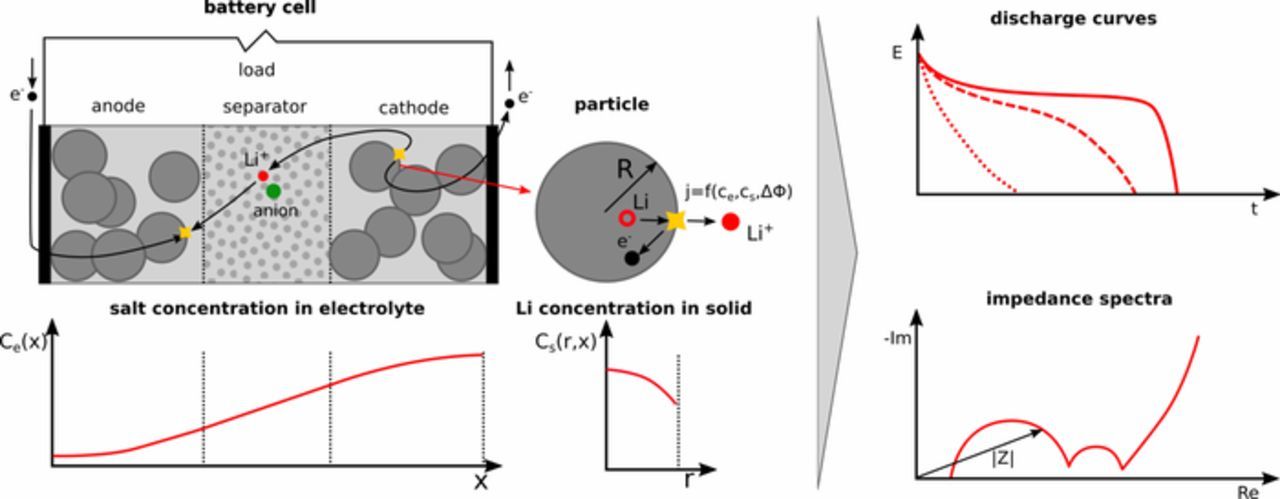
Many different approaches for the dynamical modeling of batteries exist, see for example Refs. 3 and 4, which describe the dynamic behavior of the cells, battery modules, or complete battery systems. Battery cells consist of a separator sandwiched between two electrically conductive, porous electrodes, the positive and negative electrodes, cf. Figure 1. The components are soaked in electrolyte to allow for transport of Li ions between both electrodes.
Figure 1. Schematic of a Li-ion battery.
During charging and discharging, an electrochemical or charge transfer reaction occurs at the interfaces of electrode and electrolyte, where Li ions are either reduced and intercalated, i.e. stored in the electrode particles, or the stored Li is oxidized, releasing electrons.
Besides internal variables such as concentrations and temperatures, two essential, performance-related variables of a battery are of general interest for its diagnosis and operation: the state of charge, SOC, and the state of health, SOH. SOC relates the available capacity at a given time to the maximum available capacity when fully discharging a battery:
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/165/16/A3656/revision1/d0001.gif)
SOH in contrast takes into account that the maximum capacity decreases with time due to degradation:
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/165/16/A3656/revision1/d0002.gif)
Too high charge or discharge rates or (dis)charging electrodes to extreme ratios of active material to lithium cause degradation and can trigger side reactions that lead to uncontrollable behavior. The stable operating range not only depends on electrode and electrolyte material and the structure of electrode and battery, but also on temperature, state of charge, and state of health. Further, slow relaxation processes in the cell, which may take up to hours,5,6 mean that even the recent history of operation needs to be considered. The highly complex and nonlinear interplay of these factors and that only current, voltage, and external cell temperature with no internal state variables can be measured in commercial cells makes optimal design, state estimation, and optimal operation challenging.
A battery management system is usually employed to actively determine and monitor the battery states, and to control the charging and discharging rate of the battery cells. Practically SOC is often estimated by charge counting, i.e. integrating the current withdrawn from or inserted into the cell, or by correlation of SOC to open-circuit voltage (OCV).7 Charging of Li batteries, on the other side, is often performed by simple charging protocols, which often only take limited information of the battery state into account.2,8,9
Due to the lack of insight into the battery processes and state, intuition or experimental trial-and-error design and operation of batteries do not fully exploit the potential of the battery and may even lead to failures of the battery system. Dynamic models provide insight into the battery states and allow the use of systems tools for identification of optimal battery configurations and trajectories.2,3 These models should be dynamic to account for temporal changes of state variables in the cell, provide insight into slow and fast processes, and allow for better parameterization, and for assessing and adjusting dynamic operation. A detailed discussion on dynamic processes and time constants in Li-ion battery cells and on dynamic measurements is provided in Dynamic operation, processes, and analysis methods section.
There is a wide variety of Li-ion battery models; models are chosen and tailored for specific tasks such as analysis, diagnosis, design, or operation of Li-ion batteries. The variety of models makes it difficult for newcomers to the field and for experienced researchers to identify the best model for their particular task, to understand which information and insight into the cell can be obtained from a given model, and to properly and effectively use, train or parameterize the model.
Basically three different approaches for modeling of Li ion batteries exist. Mechanistic models are based on first-principles modeling and consider the physical, electrical, and chemical phenomena in the battery. As such these models provide deep insight into the physical and chemical processes and state of the battery. Mechanistic models section discusses the versatility of mechanistic models. They can be challenging to parameterize and are more computationally demanding than other model types. Mechanistic models section also discusses the use of mechanistic models for prevention or detection of operation in fast-aging or safety-critical operational conditions. Equivalent circuit models map the ionic and electric processes inside the cell to a network of electric circuit components, such as resistors and capacitors. Despite not containing first principle expressions for electrochemical and transport processes, equivalent circuit models often can be easily adjusted to reproduce measured behavior reasonably well. Among other aspects, Equivalent circuit and impedance models section discusses their suitability and limits regarding diagnosis and operation of Li-ion batteries. Data-driven models are constructed based solely on measurement data, often exploiting techniques from time series analysis, machine learning, or artificial intelligence based approaches to describe the input-output behavior. As such, they provide only limited insights into the internal physicochemical phenomena within the battery. Data-driven modeling approaches, which are discussed in Data-driven models section, can potentially model complex behavior such as aging or other effects that may not yet be sufficiently physically understood. Such modeling, however, may require use and misuse scenarios that make the associated training and analysis time consuming and expensive.
In addition to reviewing past achievements in understanding dynamic behavior and building models to further the efficient application of Li-ion batteries, all sections provide suggestions on future directions.
Dynamic Operation, Processes, and Analysis Methods
Dynamic operation and changes in battery state
Due to the resulting change in state of charge, i.e. chemical composition of the electrodes, the electrodes continuously change state during operation. Li-ion batteries are operated by adjusting cell current or voltage. For most larger batteries, the management system comprises also active heating or cooling of the cell, which adjusts the cell temperature. Li-ion batteries can be operated highly dynamically with load (i.e., current or power) changes even in the sub-second time scale. Applications with such dynamic requirements may be found in transport10 but also for frequency stabilization of the electric grid.11 Whereas the cell can produce the required current almost instantaneously, most processes inside the battery will reach a new quasi-steady state only after a significantly longer time (see Dynamic processes and their time constants section); the cell may even need up to minutes or hours to establish a new quasi-steady state. The vastly different time scales hold especially for large cells, where a load change will trigger a change in temperature and concentration distribution inside the cell. The resulting local gradients trigger heat and mass transport processes over long distances, i.e. long times, in the cells.
Batteries are closed systems, with Li being stored into or released from the electrodes during charging or discharging. Thus, as long as the current is not set to zero, no steady state can be reached and the SOC changes. The changing current in turn results in changes of solid and liquid Li concentration and potential – and possibly other changes – over time. Also dependent on the SOC are the time constants for the Li concentration and potential to establish a new quasi-steady state after dynamic operation (see Dynamic processes and their time constants section). As such, SOC and the corresponding changes in local concentration and potential can be detected by dynamic methods. For achieving high precision, SOC estimation and prediction methods are more and more supported by dynamic measurements (see Dynamic measurement methods section) and by model-based methods, ranging from mechanistic models (Mechanistic models section) to equivalent circuit models (Equivalent circuit and impedance models section) to data-driven methods (Data-driven models section). This article provides an overview on the various dynamic models used for understanding and estimating the cell state and future developments needed to get more insight and more precision.
On the long time scale and when operating outside a specified safe operating range, the battery inevitably ages. A battery is thus a time-variant system, i.e., a system with time-dependent constants associated with aging. Aging causes changes in geometry and material properties, which in turn affects the processes inside the battery, the corresponding time constants, and the state variables. Nearly all state variables such as local concentrations and potentials thus change during aging, even when at the same state of charge. A wide range of degradation mechanisms can occur at the negative or positive electrode and in the electrolyte, which depend not only on material and cell design but also on past and present operating conditions.12 Barre et al.13 provide an overview on the degradation mechanisms and their estimation. Of high practical implication is especially the loss of available Li for reaction, i.e. the loss in the battery's capacity, and as such the SOH, caused by battery aging. The main process consuming Li is the reaction of the electrolyte at the surface of the negative electrode, which leads to a thin degradation film at the particle surface known as the solid electrolyte interphase (SEI). Its growth rate and composition is complex and depends on the past operating conditions and on cell design.14
The cell, its state, and its dynamic behavior may also be impacted by external disturbances, such as changes in the environmental temperature and external mechanical forces that lead to deformation of the cell. Mechanical deformation of the cell, but also past or present operating or environmental conditions that favor (rapid) degradation, may lead to violent reactions inside the battery that can cause the cell to catch fire and/or explode. Recently, the state-of-safety was proposed to quantify the probability that the cell remains in a safe state15 after having been exposed to certain abuse conditions; The state-of-safety accounts for various abuse conditions by multiplication of the probabilities for each abuse process. Rather than considering the fast processes occuring during such a runaway, which are highly complex, this review focuses on stable operation with and without aging. The reader is referred to the review by Abada et al.1 and the work of Kim et al.16 for the modeling of instabilities in batteries.
Most studies on dynamic battery operation and state estimation are on a systems level. They usually contain sophisticated mathematical analysis and estimation tools, but have minimal consideration of the processes and materials in batteries. Bringing together battery experts with systems experts will allow the increased use of knowledge of battery processes and dynamics for more reliable and save battery operation. Furthermore, knowledge of the demands and limits of cells regarding stable, dynamic operation will benefit the cell and material specialists as it enables them to tailor their cells for higher dynamic performance.
Dynamic processes and their time constants
The main state variables of the cell are potential and Li concentration in the solid active material and in the liquid electrolyte, as well as temperature. The states may be spatially distributed inside each of the cell's components. Their dynamic behavior depends on the local accumulation capacity, the transport to and from the location, and the sink or source terms at the location. For each state variable, a conservation equation can be formulated in the generic form:
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/165/16/A3656/revision1/d0003.gif)
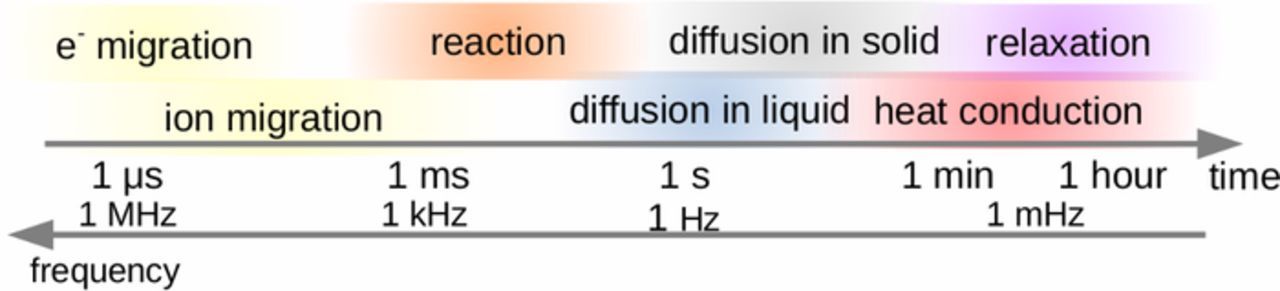
To understand the dynamic behavior of batteries better, it is helpful to extract from these balances characteristic time constants, which are typical response times that reflect the interaction of a transport or source process with the accumulation term. These time constants, which often contain material and electrode properties, may range from microseconds to hours, as illustrated in Fig. 2. The time constants may depend not only on cell design, but also on SOC, on the operating conditions – especially current and temperature – and on the SOH of and degradation processes in the battery; as such, the numbers given in Fig. 2 are approximate values. In the following we discuss the main processes leading to dynamic behavior of batteries, with special emphasis on Li-ion batteries.
Figure 2. Dynamic processes in Li-ion batteries with typical range of their time constants. Aging and degradation processes may cover wide range.
Concentration changes inside Li-ion cells are described by species conservation equations, with Li intercalation as sink or source at the electrode particle surface, and the transport processes being diffusion in electrode and electrolyte, migration in the electrolyte, and convection in the electrolyte. The latter process is usually negligible for Li-ion batteries with intercalation electrodes. Diffusion is a slow transport process; the corresponding time constant τdiff is calculated from the characteristic length of diffusion x and the diffusion coefficient D:5
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/165/16/A3656/revision1/d0004.gif)
Using the electrode thickness as characteristic length, typical time constants of Li ion diffusion are in the range of five to twenty seconds. Solid diffusion is often slower despite having a significantly smaller characteristic length – the particle radius – as the solid diffusion coefficient is very small. Time constants vary heavily with the particle size and chemical composition and properties of the active material and are typically in the range of ten to 120 seconds. For material properties that change with concentration or SOC, the time constant becomes SOC-dependent. These time constants, however, cannot explain the relaxation process of several minutes up to hours, which is observed when switching from operation to open circuit voltage.5,6 Indeed, the slow relaxation process, which equilibrates the Li concentration in the solid particles across the electrode thickness, originates from a chain of processes, mostly solid diffusion, charge transfer reaction, and liquid diffusion.
The dynamics of the potential is described by charge conservation equations, with accumulation corresponding to double layer charging or discharging, and the transport processes being electron transport in the electrodes and Li ion or counter ion transport in the electrolyte. Electric transport is described by migration only, which follows Ohm's law, whereas the transport terms in the electrolyte often contain migration and diffusion. At places, where no accumulation takes place, i.e. at all places in the battery except for the interfaces between solids and liquids, electroneutrality holds. Assuming migration as the main charge transport process in solid or electrolyte, the time constants for the solid and electrolyte may be calculated from Ref. 5 as
![Equation ([5])](https://content.cld.iop.org/journals/1945-7111/165/16/A3656/revision1/d0005.gif)
with x being the characteristic length for conduction, Cdl as the double layer capacitance, as as the specific active area, and κ as electric or ionic conductivity. Again, with x as the electrode thickness,  , and typical ionic or electric conductivities. Typical time constants are in the lower millisecond range, both for solid and the electrolyte. These time constants are much smaller than the time constant ascribed to the double layer charge/discharge experimentally, which is usually in the range of few seconds.17 This difference is attributed to two additional slower processes that affect the charge balance, which are the electrochemical charge transfer reaction as a source term and the diffusive charge transport in the electrolyte, both of which depend on Li-ion concentration. As for the case of diffusion, these time constants may depend on SOC when material properties or Li concentration change at the interface. Also, changes at the interface, such as the SEI, through which Li ions need to migrate between electrolyte and active material, will lead to additional characteristic time constants.
, and typical ionic or electric conductivities. Typical time constants are in the lower millisecond range, both for solid and the electrolyte. These time constants are much smaller than the time constant ascribed to the double layer charge/discharge experimentally, which is usually in the range of few seconds.17 This difference is attributed to two additional slower processes that affect the charge balance, which are the electrochemical charge transfer reaction as a source term and the diffusive charge transport in the electrolyte, both of which depend on Li-ion concentration. As for the case of diffusion, these time constants may depend on SOC when material properties or Li concentration change at the interface. Also, changes at the interface, such as the SEI, through which Li ions need to migrate between electrolyte and active material, will lead to additional characteristic time constants.
Temperature changes are described by an energy conservation equation, with the accumulation term containing the change in inner energy, and the energy leaving or entering being heat transport via conduction and electric energy. The change in inner energy contains the change in temperature, as well as the entropy and Gibbs energy released during the electrochemical reaction. Taking into account heat conduction as the main heat transport mechanism, the corresponding time constant can be formulated analogous to that of diffusion as
![Equation ([6])](https://content.cld.iop.org/journals/1945-7111/165/16/A3656/revision1/d0006.gif)
with the characteristic length x, the density ρ, heat capacity cp, and heat conductivity kheat. Taking electrode thickness as the characteristic length and typical values for the material constants,18 the time constant is in the milliseconds range. However, technically relevant temperature gradients for Li-ion battery cells are rather occurring along the length, height, or thickness of the complete battery. Depending on cell design, a more appropriate length is therefore either the length of the electrode or the thickness of the stack consisting of several electrodes, separators, and current collectors. In this case, the time constants for temperature of technical cells easily reach several minutes. Temperature affects most material properties, and as such time constants of non-thermal processes as well.
The slowest processes in battery cells are the degradation processes, including SEI formation. Many different degradation processes can occur in a Li-ion battery; furthermore, models for each degradation process are complex, which even more holds for formulating time constants for them. These time constants depend strongly on operating conditions. The same holds for the thermal runaway. Degradation and the subsequent thermal runaway can thus take from a few minutes to months. Last but not least, each degradation process affects the processes in the battery in a specific manner, e.g. by modifying material properties or even adding further processes, such as Li ion migration through the SEI. As such, the respective time constants may change or, as is the case for the SEI, new time constants need to be added.
Most research in the area of analyzing battery dynamics is on the system level and focuses on reproducing, predicting, or controlling batteries using dynamic models (see Mechanistic models, Equivalent circuit and impedance models, and Data-driven models sections); these models are often tailored to a given application, e.g. automotive. Publications in this area usually do not analyze the origin of the observed dynamic behavior or how to use insights on the dynamic processes for better battery design, analysis, or operation. Other studies which are discussed in Dynamic measurement methods section, focus on analyzing or interpreting dynamic measurements, such as impedance spectroscopy, and on how to parameterize models. Such research is mostly phenomenological, with resistances and capacitances being extracted by the use of equivalent circuits. An approach directed toward understanding the origin of dynamic behavior and its link to time constants was started by the group of Newman.5 In the last 20 years, there has been little systematic continuation of research on time constants for Li-ion batteries despite their potential benefit in developing deeper understanding of dynamic measurements that could be used in the design and operation of safer and better batteries. The overview in the next section is hoped to catalyze research and guide future activities in this direction.
Dynamic measurement methods
As discussed in the previous sections, batteries are frequently operated dynamically to follow load demands, and various slow and fast processes take place in parallel in the battery during such dynamic operation. These processes interfere with each other and yield a complex dynamic behavior, which is observable in the interplay of battery current and voltage vs. time. Fast dynamic processes will govern the response within micro- and milliseconds, whereas responses related to slow processes such as solid diffusion will only be observable after longer response times. The resulting overall dynamic response of the battery to a certain change in voltage or current is thus characteristic for the processes in its interior and, for a given design of a battery, its operating point, state of charge, and state of health. Thus, dynamic signals contain significantly more information on a battery's state than a (quasi-)steady state measurement point as recorded during a discharge curve, where only the voltage is observed for a certain current, state of charge, and operating point. Furthermore, dynamic measurements offer essential insights into the state of the battery during operation which otherwise can only be gained with highly expensive operando methods such as X-ray microscopy.19,20 Such methods usually also require specially designed in situ cells, i.e. they are not applicable to monitor full cells in applications.
The dynamic response of current to a change in voltage or of voltage to changes in current is therefore used not only to evaluate how fast a battery can deliver a certain power, but also to evaluate the processes leading to good or bad performance and to determine its state of charge and health. Dynamic measurements used in fundamental research on material and batteries help to understand the behavior of and processes in battery material and cells,21,22 and they are used to parameterize or refine battery models.10,23 Finally, dynamic measurements are essential parts of diagnostic tools to monitor the state of health and state of charge of batteries,17,23–29 and they can be used to design optimal battery operations, i.e. charging and discharging.30–32
Electrochemistry offers a variety of dynamic measurement methods that can be applied to batteries. The methods differ mostly in the shape of the dynamic inlet signal, which may take the form of step changes, impulses, ramps, sinusoidal signals or other defined or random profiles.33 The latter often are application-specific. The analysis of the response to a step change in current or voltage is called chronopotentiometry or chronoamperometry and yields a dynamic change of voltage or current vs. time. The instantaneous response within the first second is often used to determine internal electric and ionic resistances, as the corresponding time constant of charge transfer is in this range (see Dynamic processes and their time constants section). Slower processes follow, so that the resulting response contains – according to Fig. 2 – first the information on SEI transport, reaction, and then on diffusion and heat transport. Fast Fourier Transformation analysis of this signal may be done, if the step change is within the linear range, to extract a characteristic linear frequency response, i.e. an electrochemical impedance spectrum. Similarly, square input signals may be used.34,35
The precise and standard way to record the linear frequency response is by submitting the battery to a small sinusoidal change of current (or voltage) and recording the sinusoidal response of voltage (or current). Input frequency and output frequency ω are identical, and the phase shift ϕ(ω) and ratio of amplitude  between both signals are evaluated. Phase shift and amplitude ratio together constitute the impedance Z(ω) of the cell:
between both signals are evaluated. Phase shift and amplitude ratio together constitute the impedance Z(ω) of the cell:
![Equation ([7])](https://content.cld.iop.org/journals/1945-7111/165/16/A3656/revision1/d0007.gif)
Electrochemical impedance spectra (EIS) are frequently plotted in the Nyquist diagram for a wide range of inlet frequencies from mHz to kHz. Typical spectra often resemble a composition of – sometimes compressed or overlapping – semi-circles and optionally tilted lines, as seen in Fig. 3b. Semicircles are usually attributed to certain processes using their characteristic frequency.17,22 In contrast to analysis of step changes, electrochemical impedance spectroscopy allows a precise stimulation of processes with a certain time constant by applying its corresponding frequency value. The transition from one dominant process to a different characteristic process may be visible in the spectra via changes of the angle and amplitude. This characteristic change in slope may be better visible when applying a data processing method such as distribution of relaxation times.36 However, this method requires the use of equivalent circuit models, i.e. physically motivated empirical models (see Equivalent circuit and impedance models section). EIS have been intensively used for estimating the SOC and SOH, see e.g. Refs. 17, 23.
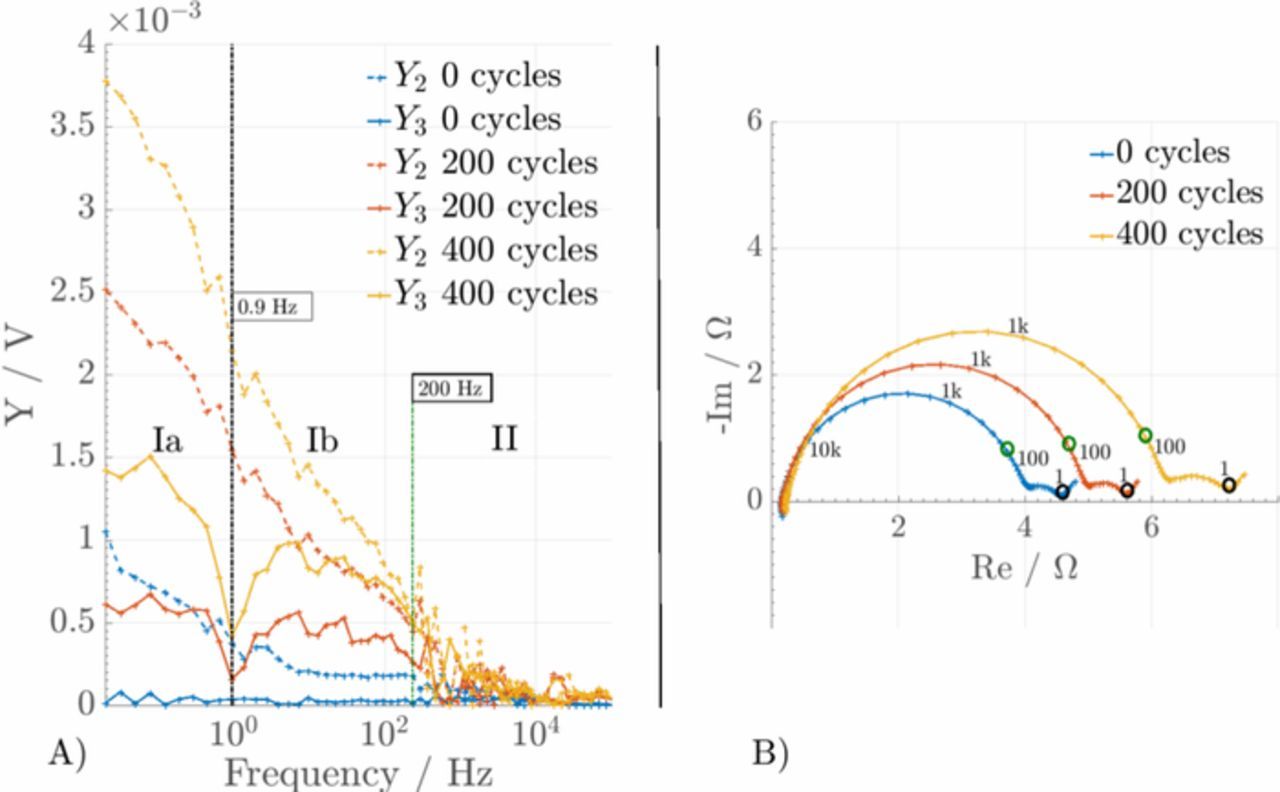
Figure 3. (A) Nonlinear frequency response analysis and (B) electrochemical impedance spectra of aged Li-ion batteries. Region Ia: Diffusion-driven processes, Region Ib: Reaction-driven processes, Region II: Transport/Interphase processes of SEI (reprinted from Ref. 22 with permission from Elsevier).
EIS is a linear analysis method, which simplifies interpretation as a wide range of tools from the field of linear system analysis is available. However, valuable information on the processes inside the battery is ignored, as the linear analysis ignores the nonlinear nature of the battery, which is reflected especially in the exponential dependence of current to potential. The complexity and large number of processes and parameters in the cell lead often to non-unique mapping of processes to features in the spectrum. As such, nonlinear analysis methods are a very attractive alternative or complement to EIS for batteries. For example, nonlinear frequency response analysis analyzes the voltage (or current) resolving from subjecting the cell to a sinusoidal input of large amplitude. This signal contains not only sinusoidal responses Y1 with the same frequency as the inlet frequency, but also higher harmonic responses at integer multiples of the inlet frequency (Y2, Y3, ...). Typical frequency regions can be correlated to that of EIS, as illustrated in Fig. 3 for cycle aging of Li-ion batteries.22 A comparison with EIS reveals that nonlinear frequency response analysis offers essential additional information via a clear distinction between linear processes such as transport through SEI (Region II) and nonlinear processes (Region I). Our recent studies show that the method may distinguish between different types of aging of a cell, which is not the case for EIS.25 Nonlinear frequency response analysis is thus especially attractive and useful for assessment of the SOH and safety of a given battery. However, as the method is relatively new, very few studies and experimental data is available and there is little knowledge of how to interpret the spectra. Wolff et al.37 were the first to simulate such spectra with mechanistic models and showed via parameter variation the effect of the single processes and parameters on the spectra. Future model-based research is recommended for a quantitative and qualitative understanding and thus interpretation of the spectra, including also aging effects.
Further methods used to investigate nonlinear cell behavior are the dynamic EIS and the dynamic pulse method. Both methods provide current-dependent impedance spectra. With dynamic EIS, the sinusoidal excitation is superimposed by an offset current. This offset current leads to a nonlinear voltage response, which can be detected and interpreted using the methods of the EIS.38 In analogy to dynamic EIS, a pulse can also be superimposed with an offset current. The advantages and disadvantages of the two methods are discussed in Ref. 39.
Various dynamic operation scenarios, such as automotive driving cycles or dynamic charge strategies, may be used for training and evaluating model-based SOH and SOC estimators. For example, Charkhgard et al.40 estimated the SOC by analyzing the dynamic pulse charging and the resulting noise, He et al.41 used a commuter driving cycle and a resistance-capacitor model with data-driven methods to estimate the SOC, while Klein et al.42 and Moura et al.43 used a driving cycle and a mechanistic model with observer to estimate SOC and SOH and monitor cell states such as temperature. Few of these studies investigate internal state variables and processes during dynamic operation, and most studies use artificial experimental data generated from the model itself, leaving room for future studies.44
Mechanistic Models
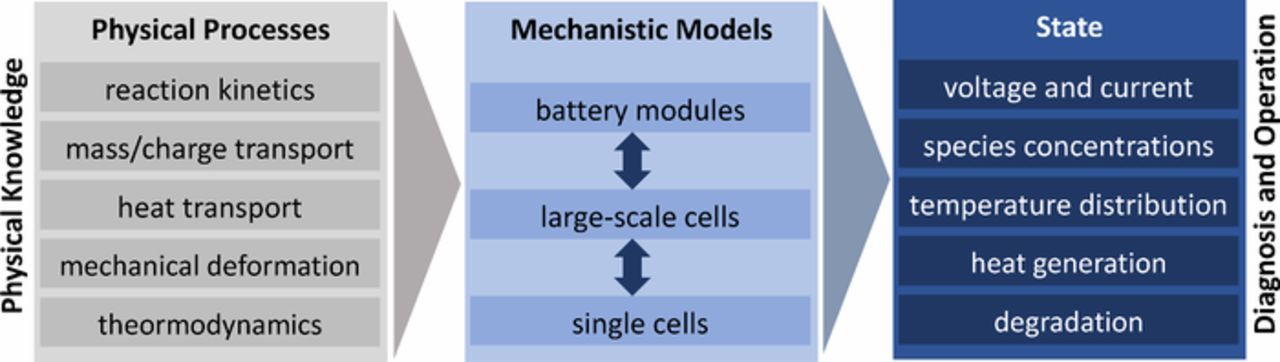
The properties of battery cell components, especially of active material and electrolyte, and of their geometrical arrangement determine the properties and the dynamic behavior of battery cells. Mechanistic models of Li-ion batteries aim to describe with mathematical equations, based on physical and chemical knowledge, the processes occurring in or between the components and the resulting battery performance and state, e.g. SoH or SOC. The major processes that are covered in models thus are electrochemical reactions; mass, charge, and heat transport; and unwanted chemical side reactions or deformation of components leading to degradation of cell performance. The local accumulation of chemical species, charge, and energy, and the degradation and deformation processes finally lead to the dynamic behavior of a cell (details on processes and dynamics see Dynamic processes and their time constants section). The scope of the mechanistic models is illustrated in Fig. 4. Experimentally, only a small portion of the dynamics can be directly measured, whereas mechanistic models can reveal the dynamics of all processes modeled. In addition, they can give an insight into the underlying causes and interactions and thus allow the interpretation of the experimentally observed dynamic behavior (see also Dynamic measurement methods section). This insight and the reproduction of the experimentally observed behavior is, though, limited by the physico-chemical phenomena covered by the model, and by the accuracy of the corresponding mathematical descriptions and model parameters. Model parameters that cannot be measured directly, e.g. using porosimetry, or estimated theoretically, e.g. using quantum-chemical simulations, need to be determined by adjusting the model output to experimental cell behavior. Determining highly accurate parameters is a cumbersome and tedious process,45 and uncertainties and ambiguities in parameter estimation may lead to systematic and large errors in simulation output, and as such in the conclusions drawn regarding performance and state. This problem is especially severe when the model should be used for control or state estimation of commercial cells, as for commercial cells often barely any information is known on the components and their parameters. Besides the model formulation and parameter estimation, also the numerical implementation of the model and the simulation process determine accuracy of the simulation result.
Figure 4. Scope of mechanistic models: Understanding, prediction, reproduction and control of physical states and behavior of Li-ion batteries.
As a rule, a model that describes all dynamic processes at all levels is neither feasible nor usable. Mechanistic models of batteries always need to be tailored to the purpose of usage, e.g. the scientific question to be answered or the quality and speed with which a state or behavior of the cell should be estimated, predicted or adjusted.
Models for analysis and diagnosis purposes employ detailed descriptions of the phenomena and processes to maximize insight into the property of interest, and thus are often multidimensional, multiphysical, and computationally slow. For control and optimization purposes, usually simplified and computationally fast models with a limited description of the phenomena are developed. Nevertheless there is also a need of sufficient physical insight for operation, e.g. for a state estimator, and of fast computation for diagnosis, e.g. to enable parameter identification. Decades of research have aimed to develop new models or model modifications which allow more efficient computation of phenomena, more detailed physical insight, better resolution of heterogeneity, or simulation of actual geometries. The next section provides an overview of the most commonly used mechanistic model approaches for the simulation of dynamic processes in Li-ion batteries, their fields of application and future trends. The discussion focuses on particular application of those models for operation and diagnosis and incorporate kinetics, thermodynamics, and degradation.
Single-cell models with homogeneous electrodes
Battery modeling often focuses on analyzing and optimizing the performance of a single homogenized electrode-separator-electrode unit, i.e. a single cell. The most commonly used mechanistic model for single cells is the pseudo-two-dimensional (P2D) model.46 Good agreement to experimentally recorded discharge curves and its computational efficiency, which allows simulations from short to long time scales, makes the P2D model a good compromise for a wide range of applications. Figure 5 illustrates the idea of the P2D model, the main processes implemented in the model, and typical simulation outputs, i.e. discharge curves and impedance spectra.
Figure 5. Illustration of the pseudo-two-dimensional battery model and its output.
P2D models usually include lithium-ion accumulation and diffusion as well as electrical charge transport in one dimension x in the direction of electrode thickness, electrochemical reactions at both electrodes, and the diffusion and accumulation of intercalated lithium into the active material particles in a second dimension r. The model allows the modeling of electrochemical performance and SOC, while also providing important insights into the cell state via local potential, concentration, and reaction rate. In the P2D model, electrodes are modeled as homogeneous multiphase systems based on porous electrode theory.47 The actual scope depends on the particular implementation, and thus various models with similar structure are still widely used and under development. In the following, some common extensions and modifications are discussed, which include chemical and mechanical degradation, temperature accumulation and charge accumulation, i.e. double layer charging, at the electrochemical interface.
Inclusion of the rapid double layer (dis)charging process at the particle surface48 is not required for modeling constant charge/discharge operation, as the double layer is in quasi-steady state after very few seconds (see Dynamic processes and their time constants section). However, the inclusion of this process does enable simulation of EIS49 and nonlinear frequency response.37 To simulate EIS, two distinct approaches exist; either equations are transformed into the frequency domain and solved analytically,50 or the model is evaluated in the time domain and simulation output is transferred via fast Fourier transformation. The latter approach can be also used for nonlinear frequency response analysis.37 The double layer charging/discharging process becomes relevant when highly dynamic current profiles including ramps or step changes are applied and the response in the first seconds is of interest. Besides Legrand,51 who included the double layer to model ramps, most other works neglect this voltage dynamics. The double layer is also ignored for models that simulate driving cycles of electric vehicles, as those models focus on a larger time scale.42,43
Most of the processes, such as diffusion and electrochemical reactions, strongly depend on cell internal temperature. Moreover, heat generation within the cell is the result of multiple physical processes (see Dynamic processes and their time constants section). Besides current and voltage, the cell temperature is the only variable that can be manipulated from outside the cell during operation, and it has a significant impact on performance, safety, and degradation. During operation of Li-ion battery cells in many applications, such as electric cars, the temperature frequently changes due to cell internal heating, environmental conditions, or active cooling systems. Therefore, an accurate consideration of temperature dependency and heat generation is usually essential for diagnosis and control, and may only be excluded for diagnosis of small cell formats and slow charging under temperature-controlled laboratory conditions. Mechanistic models considering temperature and heat generation require an extension of the classical model equations and parameters.52,53 Temperature dependence of parameters such as for diffusion coefficients or reaction rates usually enters the models via Arrhenius terms using activation energies, which can be estimated for instance by pulse tests at various temperatures and current rates.54 As most transport and reaction parameters are temperature dependent with different activation energies, identification of a unique set remains challenging.
Degradation of Li-ion batteries inevitably occurs during operations. As degradation affects many processes and parameters in the cell including time constants and quasi-steady-state cell performance; extending P2D models toward degradation is essential. Most model extensions regarding degradation consider the solid electrolyte interface (SEI) at the negative electrode, which results in a decrease in capacity and SOH, and in increased ion transport resistance. The major impact of the SEI film on the dynamic behavior of Li-ion batteries can be covered by implementing an additional resistance at the surface of the particle55 and a shift of open circuit potentials of the electrodes. Simulation of the impact on EIS requires using models with an additional double layer capacitance.49 Such models are applicable for the simulation of battery behavior for different surface film thicknesses, and may in future be used to estimate the film thickness based on measurement data. However, simulation of transient changes of battery performance over a long time scale, as needed to predict cell aging, requires the implementation of a film growth. The growth rate decreases with increasing film thickness, which has been implemented as a diffusion-limited56 or kinetic-limited57 process. However, the actual physical causes are still under discussion. Due to volume change of active material during lithiation/delithiation, active material particles and electrode structures, are stressed mechanically, which can lead to cracks in particle and electrodes and loss of active material.58,59 A combination with SEI modeling60 is of high interest, because the volume expansion can cause SEI destruction and reformation. To better understand degradation in Li-ion batteries, future studies should focus on the interaction of various degradation processes and impact of local conditions, such as local current densities and temperature. However, distributed or multiscale models seem to be more suitable for this purpose, as many degradation processes originate or spread from heterogeneity in the cell (see below).
P2D models describe physicochemical processes by using more than fifteen parameters containing geometric information, such as layer thicknesses, porosity and average particle sizes, material properties such as diffusion coefficients and conductivities, and further parameters such as active surface area, exchange current density, and initial concentrations. Many of these parameters are available at the manufacturer; while geometric information is more easy to acquire, other parameters are more intricate, and may not be directly measurable. Further, many parameters have a strong nonlinear dependency on temperature, which requires careful parameterization of the model with electrochemical measurements, such as charge/discharge curves61 or electrochemical impedance spectroscopy.62 Half cell measurements allow to separately measure – and thus parameterize – negative and positive electrodes, but they are challenging. Indeed, the uniqueness of the identified parameter set is debatable, especially if parameter estimation involves a high number of parameters that are jointly identified using only discharge curves or only electrochemical impedance spectra. Furthermore, parameters may be sensitive to changes in the manufacturing procedure.63 Already small changes in the production process of electrodes were shown to strongly impact internal electrode parameters identified by the model.64 In addition, measurement errors or fluctuations caused by the production process can cause quite large uncertainties in parameters and as such in subsequently identified parameters and the predicted performance of the model. Uncertainty quantification and global sensitivity analysis are excellent methods to identify how accurate parameters are required to be measured or adjusted in production.63,65 Identifying more accurate model parameters improves the accuracy of the predicted model results. Furthermore, knowing which battery parameters to focus on during production and adjusting them more accurately will allow the minimization of performance fluctuations occurring during production. More sophisticated parameterization and uncertainty analysis thus are important future research directions. Better established and reliable methodologies for parameterization furthermore will make switching from easily parameterizable (semi-)empirical models, such as equivalent circuit models, more attractive.
P2D models are often an adequate platform to better understand cells, e.g. the effect of cell design and operation on performance and to get an insight into the limiting processes or process interactions. P2D models are too lumped or simplified to describe processes at the molecular level or their the complex electrode structure and composition. On the other hand, their computational cost is often too high and further simplifications are usually done before application of model predictive control or state estimation.32,66,67 Several approaches for model reduction of the P2D model are possible. Reductions can be motivated either by the need for computationally faster models or by focusing on only on those features that the model is required to have for a certain task. A widely applied reduction is the single-particle model, where the distribution of current, voltage, and concentration along the electrode layer is neglected, and the layer is represented by a single particle, respectively.68 This approximation is good as long as electrolyte concentration or electrical potential is homogeneous along the thickness of the electrode, which is often the case for slow charging/discharging, thin film electrodes,69 or fast ionic and electrical conductivity. A further frequently applied model reduction is the approximation of the diffusion process inside the active material. Approximations can be a polynomial68 or analytical.70 Alternatively, the concentration gradient may be neglected, which can be sufficiently accurate for active materials with fast diffusion or short diffusion pathways, i.e. small particles. In general, thermal aspects,71 chemical72 and mechanical degradation73 and double layer capacitance50 can be considered comparable to P2D model. With the physically motivated simplifications as discussed here, computational efficiency of the model can be increased considerably. Another possibility is to reformulate the model implementation and numerics to decrease computational cost.74–76 This approach may facilitate their application for operation in a real-time optimization in nonlinear model predictive control32,75,77
Models for large-scale cells and battery modules
Actual technical batteries are hierarchical structures that are composed of modules, which are composed of large-scale cells connected in parallel or sequence; the cells may again consist of several stacked single cell layers or may be composed of just one long rolled or folded electrode-separator-electrode entity. The composition and geometry of the cells and modules are tailored to the application and considerably impact performance and dynamic behavior of the battery. Uneven concentration and temperature distributions are typical features of large cells, causing complex dynamic behavior and bearing the risk of producing local sub-optimal or even safety-critical conditions. The distributed nature of the battery makes intuitive battery design and operation difficult, which holds even more for dynamic operation, and thus favors the use of mechanistic models with high spacial discretization.
3D simulation of the electrochemical processes in larger cells is computationally expensive and numerically challenging. To overcome this problem, the battery model can be decomposed into a 1D electrochemical model for describing the processes in the cell, coupled to a 2D resistor network and a 3D thermal model.78 This approach is motivated by the fact that transport of electrical charge and heat conduction are major processes, which are on a significantly larger length – and thus time – scale than the electrochemical processes taking place over the thickness of one electrode or electrode-separator-electrode entity. Such multi-physical models enable dynamic simulation of the interaction between thermal, electrical, and electrochemical processes. Various tailored models have been developed to analyze spacial distribution of degradation, e.g. SEI growth,79 thermal and mechanical80 abuse, several cells in series81 and whole battery packs including the cooling system.82 Moreover, these models can be used to investigate the impact of dynamic operation of the batteries on heat generation,83 which showed that uneven heat generation in battery packs are most critical with cycling in narrow state of charge ranges and pulse loading and may lead to uneven load of the battery, localized heating, and thermal runaways. In general these models are applied to aid design of battery packs, their temperature management, and optimal operation strategies to improve performance, life time, or safety. However, for an online prediction or state estimation for control purposes, the computational cost needs to be reduced significantly. Global sensitivity analysis on such high dimensional electrochemical models can indicate which parameters most affect performance or safety critical conditions, and may thus motivate future model reduction.84
Models with heterogeneity in electrodes
Porous electrodes in most battery types, such as Li-ion batteries, are highly heterogeneous systems that have pore and particle size distributions, and their composition and arrangement inside the electrode is heterogeneous as well. In P2D models, this heterogeneity is homogenized based on porous electrode theory. Even though homogenization might often be sufficient for reproducing the overall performance of a battery, it is insufficient for diagnosing and predicting electrode performance for specific structures and for understanding certain degradation or dynamic effects triggered by local heterogeneity, e.g. local current densities or temperatures. As a consequence, a few works have come up in the last years that include heterogeneity in electrode models. Further, some key processes in batteries are on very small length scales, which cannot be modeled by continuum approaches, but affect the overall macroscopic processes. Examples are atom-to-atom interaction in degradation reactions leading to SEI film growth. Understanding these processes and their effect and interaction with other processes and cell performance would enable knowledge-based design and control with the aim to avoid degradation and failure, and to increase energy or power density or fast charging. Understanding is barely possible using only experiments, so mechanistic models covering these phenomena are essential. Modeling of both aspects, i.e. consideration of inherent heterogeneity of battery electrodes and meso- and nanoscale processes, that determine the overall dynamic behavior are discussed below.
In general, explicit and statistical heterogeneity can be distinguished. Few models explicitly model the transport and reaction phenomena in consideration of the microstructure in 2D85 and 3D;86 microstructures may be experimentally recorded by computer tomography or stochastically generated.87 Since the resolution of the particle compartment in an electrode is computationally very expensive and numerically challenging, in some models only a representative part of the microstructure is simulated using surrogate models and is either directly88 or indirectly89 coupled with a homogeneous model. Further, models are available that consider details about heterogeneous particle particle interaction and electron transport to gain a more accurate understanding of the impact of particle properties such as size- and volume-fraction distribution.90 Those models can be applied in diagnosis and design of those structural aspects, which is not possible with homogeneous models, where aspects are reduced and concentrated by application of effective parameters. Of special interest are models that allow the study and reproduction of the multiple solid phases during charge and discharge, and the resulting uneven particle-by-particle (dis)charging in an electrode and hysteresis behavior;91,92 the single-particle model is found not to reproduce the observed hysteresis behavior.93 Whether multiple solid phases occur is strongly material dependent, with these citated studies focusing on iron phosphate cathode materials. To avoid explicit consideration of complex structures, efforts have been made to consider the particle size distribution as statistical property in homogeneous electrochemical models to simulate performance94 or degradation.95,96 Using such multi-particle models enables a better agreement of simulation results in particular for higher discharge rates.97 This approach has been used to identify the change of particle size distribution due to aging of the cell with the aid of EIS simulations.96 The application of population balances to model a continuous change of particle size distribution has been demonstrated,95 and it revealed that large and small particles are stressed differently due to strongly varying local charge. Such information could be used to predict the lifetime of batteries for a given operating profile. In general, models including heterogeneity are computationally more expensive compared to homogeneous models, but enable a more detailed insight for diagnosis. As such, their value and limitation for estimating SOH and SOC needs to be investigated further. Statistical heterogeneity or surrogate models can nevertheless enable to include this information with a reasonable computational effort.
Besides the inherent heterogeneity of electrodes, several physical processes on meso- and nanoscale show complex nonideal behavior and strongly interact over multiple scales, which is often insufficiently considered by homogeneous models. Multi- and mesoscale models have been developed to enable a more accurate consideration of these phenomena than the previously described lumped models can do. Models have been developed to simulate the structure of the electrochemical double layer at non-equilibrium conditions, which enables consideration of its structure on the redox reactions.98 In particular, as soon as a considerable amount of charge is stored in the electrochemical double layer, e.g. in electrochemical supercapacitors, the three-dimensional structure of the electrochemical double layer should be taken into account.99 Other mesoscale models have been applied to investigate the transport of ions and electrons in solid material, which is used to understand material specific limitations and enable a tailored design of material structures to achieve higher performance.100 Diffusion coefficients are often considered as constant in homogeneous models; however, mesoscale models101 reveal that diffusion coefficients in solid material can vary in orders of magnitude with concentration. The change of small sized pores in porous electrodes can significantly impact the performance of batteries, which can be predicted using kinetic Monte Carlo simulations.102 In context of chemical degradation, similar techniques have been applied to investigate passivation of the negative electrode surface through heterogeneous side reaction.103 Such models can be further directly coupled to homogeneous cell models though multiscale simulation and reveal the interaction between macroscopic properties, such as particle size, and atomistic reaction mechanisms, e.g. SEI growth.104 Meso- and multiscale models are currently underestimated compared to their importance for battery operation, diagnosis, and design. Further, these techniques allow consideration of first-principles information gained by molecular or density functional theory simulations. This approach enables part-wise parameterization of models; however, ab-initio simulations mostly consider ideal surfaces and it remains a challenge for the future to get accurate parameters for technical electrodes.105 In combination with multiscale algorithms, this approach enables simulation of dynamic processes over a wide range of length and time scales. There are promising experimental and theoretical tools and concepts available for understanding detailed chemistry and transport phenomena in batteries so we expect increasing application in the future.
Equivalent Circuit and Impedance Models
A common phenomenological approach to simulate the dynamic behavior of cells or battery systems is the usage of equivalent circuit models (ECMs). These models consist of simple electrical elements which represent the dominating electrochemical processes within a cell. The number of model parameters can be reduced by omitting or lumping electrochemical processes and physical effects; this holds especially, if they occur at similar time constants, where they may not be separated with dynamic experiments. As such, ECMs are mainly used to simulate key performance parameters such as power, energy and heat generation under a wide range of operating conditions. Furthermore, ECMs can easily be scaled up to battery up to system models.
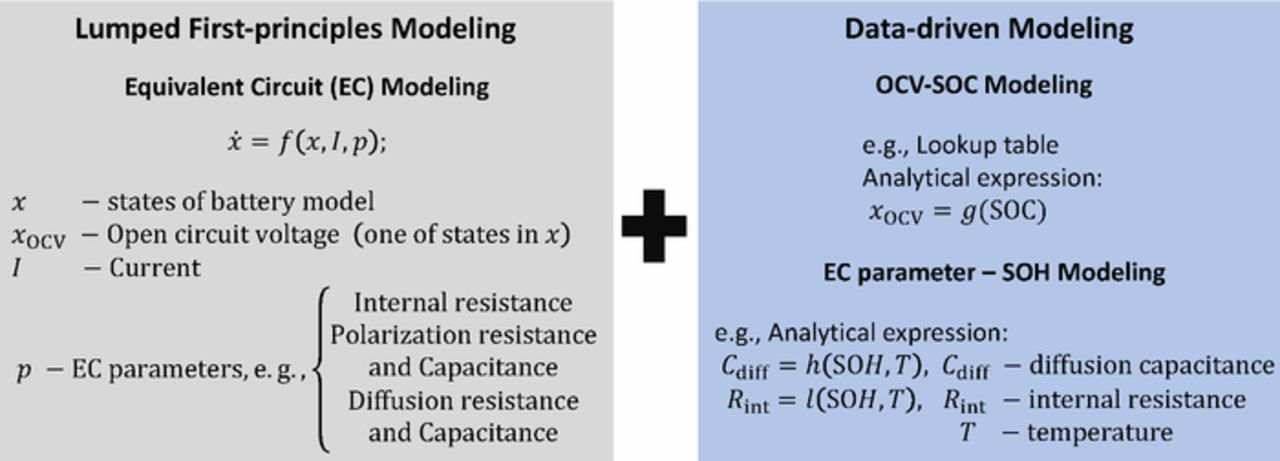
ECM can be classified in approaches containing elements that reproduce physical electrochemical processes and effects, and in approaches that don't. In models without electrochemical considerations the electrical cell behavior is usually described by an arbitrary number of RC elements, where each element contains a resistance (R) and capacitor (C) connected in parallel. Usually the impact of an RC element is independent from other RC elements; thereby they have no interaction with each other. This allows an extremely fast calculation and simple implementation. Possible fields of application including online parameterization of these models are discussed in detail in Simple and phenomenological equivalent circuit models section and Fusing data-driven modeling with other model types: grey box modeling section. However, due to the lack of electrochemical relations, interpolation and extrapolation with the model and its parameters under all operating conditions are not possible or at least only up to certain extent.
In contrast, an electrochemically motivated ECM that contains known physical dependencies enables interpolation and extrapolation of model parameters. These models can be categorized into models with spatially lumped and distributed parameters. In addition, elements and equations with fractional derivatives are typically used for description of porous structures. The electrochemical ECMs are usually assigned to the cell components which are anode (negative electrode), cathode (positive electrode), electrolyte, separator and current collector.106,107 The advantage of the ECM approach is that the measured current or voltage response of the cell corresponds to an averaged physical quantity of all parallel processes across all particles with the respective current and concentration distributions. This leads to a considerable reduction of model order. Ecker108 presented that even half cells, i.e. test cells with only one technical electrode, may be simulated by ECMs using a more detailed description of the electrodes. Furthermore, the author simulates the particle to particle interactions and the associated current and concentration distribution in the electrodes. The model approaches presented and discussed in the following differ considerably in terms of measurement, parameterization and implementation as well as computing time.
The quality of the model depends directly on the quality of the measurements. Cell effects which account for only a small proportion of the total cell behavior, are usually neglected as they often cannot be detected with the present measurement methodology and measurement technology. This means that an increase in the quality of ECMs can only be achieved by further development of the measurement methodology and measurement technology.
Simple and phenomenological equivalent circuit models
The most popular way to describe and reproduce battery dynamics is by a simple ECM. In the simplest case, ECMs consist of an SOC-dependent voltage source in series with a resistor and one or more RC circuits.109–111 This choice is primarily due to the early prevalence of models for battery management systems for portable electronics, where the approximation of the battery model with an ECM is adequate. This modeling approach was then extended to Li-ion batteries for automotive or similar energy storage applications. However, direct application of equivalent circuit models for battery-management-systems can lead to two drawbacks. First, the theoretical basis for ECM is often based on the response of the battery to a low-amplitude ac signal. Due to the nonlinear battery dynamics for large currents (see 2.3), these ECMs have limited prediction capabilities for high power applications such as in the field of automotive applications, where higher accuracy is required compared to portable electronic applications. The standard ECM can be extended by making the circuit parameters depend on SOC and temperature, or even the applied current.112–116 However, this approach highlights the second drawback, wherein a large number of parameters is needed to fit the extended ECM. Furthermore, since these parameters turn into mere fitting parameters for the model, the physical intuition behind the meaning of these parameters in an ECM is lost.
A more empirical approach is presented in Refs. 117–119 where state space models of the battery are used together with state and parameter estimation algorithms. Further developments of parameter estimation for battery management systems of hybrid electric vehicles are reported in Refs. 120,121 While this approach seems promising at first, the same drawbacks as in ECM are encountered: lack of extrapolation capability due to the simplistic model, and lack of physical relevance of the model parameters.
Since simple ECM models do not contain physical or electrochemical models, their parameters are typically time constants. Often pulses are used to determine these parameters. The parameters are stored in lookup tables or polynomial without physical relations.122 Thus, an interpolation and extrapolation is only possible to a very limited extent. It is necessary to point out that even if the extrapolation capability of ECM can be further improved, such a model often does not provide any insight into internal physical processes occurring inside the battery as can be offered by mechanistic models. Thus, models covering the processes in the battery more detailed are advantageous if increased performance and battery life are critical factors, such as in automotive and energy storage applications.
Equivalent circuit models based on electrochemical processes
ECMs that are derived from electrochemical effects and processes are usually used to optimize the design of battery systems considering energy content, cooling and performance as well as for concept evaluations. Identification and parameterization of the individual processes as well as model order reduction are mainly carried out by analyzing EIS in the frequency domain or by using the distribution of relaxation times method. However, the determination of highly precise and reliable measurement curves is challenging and requires a lot of experience, as these curves are influenced by many disturbing effects. In order to minimize errors, an optimal combination of measuring and evaluation methods must be selected (see Dynamic measurement methods section). Using impedances always requires paying attention to the amplitude of the current signal to prevent nonlinear responses and damaging of cell. In addition, there is always a compromise between the amplitude of the excitation signal and the resolution of the measuring system. A drawback of evaluation with the distribution of relaxation times method is the necessary filter. The filter is essential to separate the individual processes more clearly, but it may disturb or distort the results and resulting time constants.123
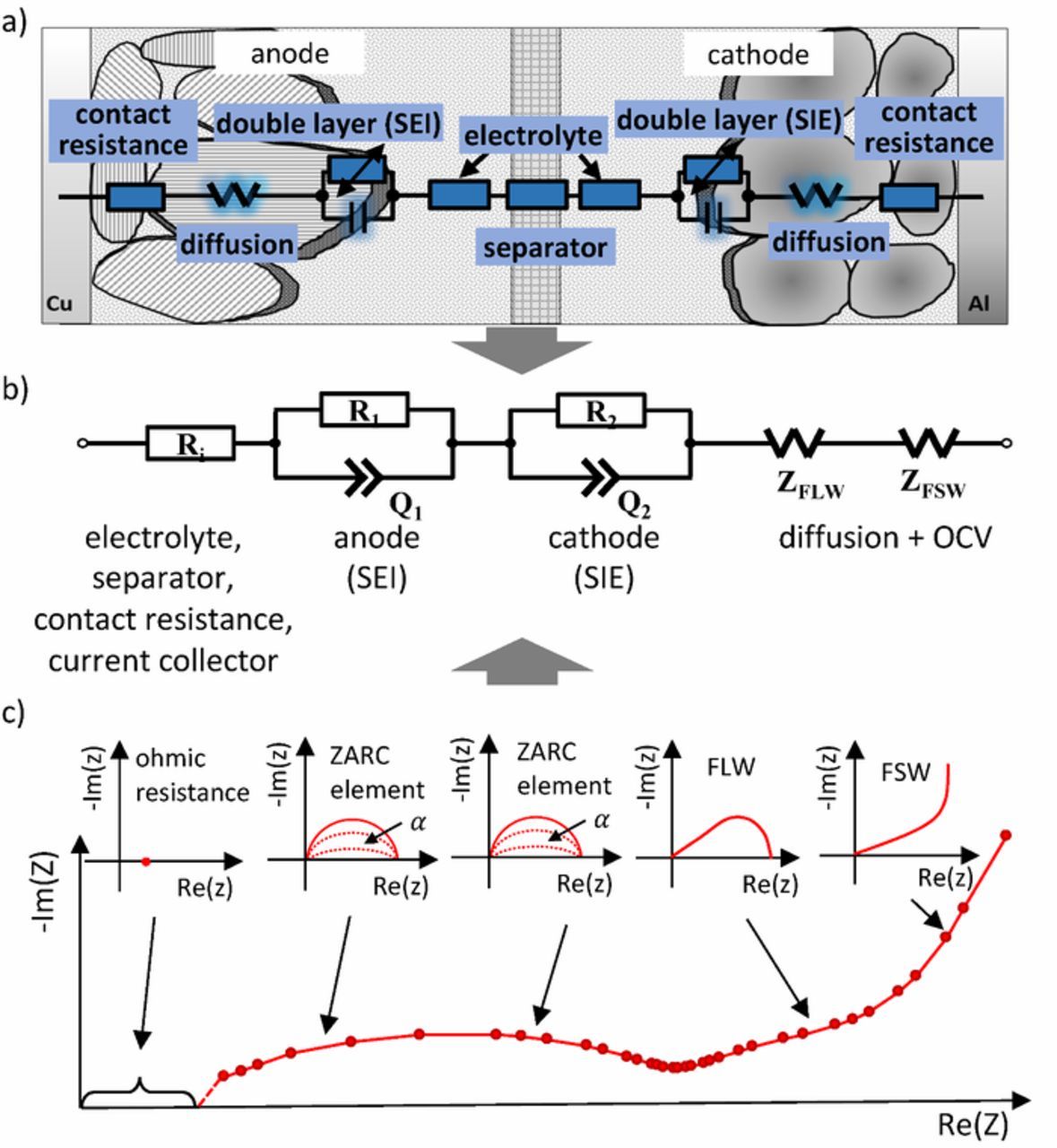
Figure 6 shows a typical procedure for creating an electrochemical ECM. The process incorporates and combines a variety of different ECM components and has been set up to contain elements that cover the main dynamic processes in cells and reproduce its cell behavior as done in Refs. 124–128 The starting point of the modeling is a sectional view of the cell (Figure 6a). There, electrical elements are drawn representing the physical effects and processes. In the next step, the model structure is created using the recorded cell impedance (see Figure 6c) and the model order is reduced by combining the elements.
Figure 6. Example for ECM components and physical equivalences, a) sectional view of the cell with physical phenomena reproduced by electric circuit elements, b) resulting ECM consists of a resistor, two ZARC elements modeled by an ohmic resistance and a parallel connected constant-phase element, a finite length Warburg (FLW) and a finite space Warburg (FSW), c) cell impedance spectra with the respective decomposition into electric circuit components.
The ECM shown in Figure 6b is a typical and flexible core model, which is often a good basis for EC modeling. An advantage of the presented approach is the modular design, which allows an extension to further cell effects, such as hysteresis, reversible heat generation and temperature dependence of the OCV (entropy change) as well as diffusion-limiting effects. In contrast to ECMs with a separate view of the negative and positive electrode, no destruction of the cell is necessary for the parameterization. The underlying reason is that ECMs with a differentiating view of electrodes need a separate measurement of negative and positive electrode. This can be done at cells where a reference electrode is installed inside as done in Ref. 106 It should be noted that it presently is challenging to guarantee that the reference electrode itself does not influence the measurement. The most important disturbances are the long-term stability of the reference and the change in the current density distribution within the cell due to the additional reference electrode. In order to prevent the influence of reference electrode, a symmetrical cell (negative electrode-negative electrode or positive electrode - positive electrode) can be constructed. The problem here is that a cell opening must be performed at a minimum of 50 percent SOC to remove the electrodes and to build a full chargeable cell. Though the here discussed ECMs are physically motivated, parameterization in general is conducted without taking into account measured material parameters. This is in contrast to mechanistic modeling, where physical parameters may require extensive and complex measurements, such as porosity or microscopy (see Single-cell models with homogeneous electrodes section).
The elements of the ECM and their correlation to physical processes as shown in Figure 6 can be combined easily to reproduce the features of the experimental impedance spectra in the frequency domain. However, the transformation of these elements into the time domain is not trivial. Possible approximations and transformation into the time domain are discussed below. The dynamic processes presented in Dynamic processes and their time constants section are usually condensed to four processes related to potential losses, i.e. impedances, when setting up ECMs, as they are easily identifiable in the impedance spectrum. These are the inductive losses of metallic elements in the cell, the ohmic resistance as the sum of electrolyte, separator, current collector and electrical conductivity of the active material, the double layer and charge transfer effects at the solid electrolyte interfaces of the electrodes and the ionic mass transport within the electrodes.129
To describe the dynamics of the double layer and the charge transfer, an RC element is often used. A closer look at the EIS and distribution of relaxation times method reveals that a simple RC element does not adequately reproduce the observed depressed semicircle, which is a characteristic feature for porous electrodes. In many cases, the RC element is extended to a so-called ZARC element. It consists of an ohmic resistance and a parallel connected constant-phase element, which allows a representation of the porous electrode structure:
![Equation ([8])](https://content.cld.iop.org/journals/1945-7111/165/16/A3656/revision1/d0008.gif)
The parameter α is a function of porosity, R is the charge transfer resistance and Q is the double layer capacity. Equation 8 cannot be transformed into the time domain with a finite calculation rule. Many different approaches to approximate transformations do exist. In Ref. 130 a good agreement can be achieved by using 3 or 5 RC elements for reproducing spectra with a range of α from 0.6 to 1. The charge transfer is reproduced with an ohmic resistance R which has a temperature dependence according to Arrhenius and also has an SOC dependency.123,128 For currents causing a voltage drop of more than 25 mV at room temperature, the nonlinear current-voltage relation must be taken into account. This leads to a reduced charge transfer resistance at high currents, which is usually described by the Butler-Volmer equation. The current dependence of the resistance can be determined by the change of the semicircles in the impedance spectrum. In the literature, often non-physical parameters for the cathodic and anodic charge transfer coefficient as well as the number of electrons involved in the electrode reaction were determined so far, leaving room for further improvements.
Fick's first and second law can be used in order to describe the usually slow Li-ion diffusion within the electrodes (see Mechanistic models section). The resulting equation for a semi-infinite diffusion layer is called Warburg impedance. In the frequency domain, the Warburg impedance is a line with a slope of –45 degree. Two additional Warburg elements can be formulated under idealized conditions and limited diffusion: finite length Warburg and finite space Warburg.129 The finite length Warburg can be derived directly from Fick's laws with an ideal reservoir and a diffusion layer between it and the surface. At very low frequencies the finite length Warburg has the behavior of an ohmic resistor. In opposite to the finite length Warburg, the finite space Warburg has a fixed amount of electroactive substance. This leads to a capacitive behavior at low frequencies. In Refs. 106,127 the three basic Warburg elements are presented and discussed in detail. For diffusion with non-ideal boundary conditions, the Warburg elements can be extended and generalized. The influence of the pore geometry and the impacts of the particle distribution on the diffusion behavior of a cell is investigated in Refs. 131,132 Furthermore, 1-, 2- and 3-dimensional diffusion can be classified.106 For the use of Warburg elements in ECM, a transformation into the time domain is necessary. A common transformation approach uses a transmission line model consist of an RC ladder network. It can be shown that the Warburg elements and transmission line models lead to the same mathematical formula.125 Transformation approaches into the time domain for all diffusion types are presented in Refs. 106,107,133,134 The Warburg elements can be parametrized by using the impedance and the OCV slope. The behavior of an electrode can be modeled by connecting the double layer model with the diffusion elements. A common approach to describe the electrode behavior is given by the Randles model.135 For real cells and time constants a serial connection of the ZARC element with the Warburg element is possible (see Figure 6) which leads to good agreement to the Randles model.106
With ECM aging effects and processes can only be calculated and predicted to a limited extend. The main reason for this is that aging is related to a series of different factors, which have are complex influence on the cell performance and their impacts on cell impedance are not understood well yet. In Ref. 136 the effects of artifical aging of cells under laboratory conditions on the impedance and the extracted ECM parameters are investigated.
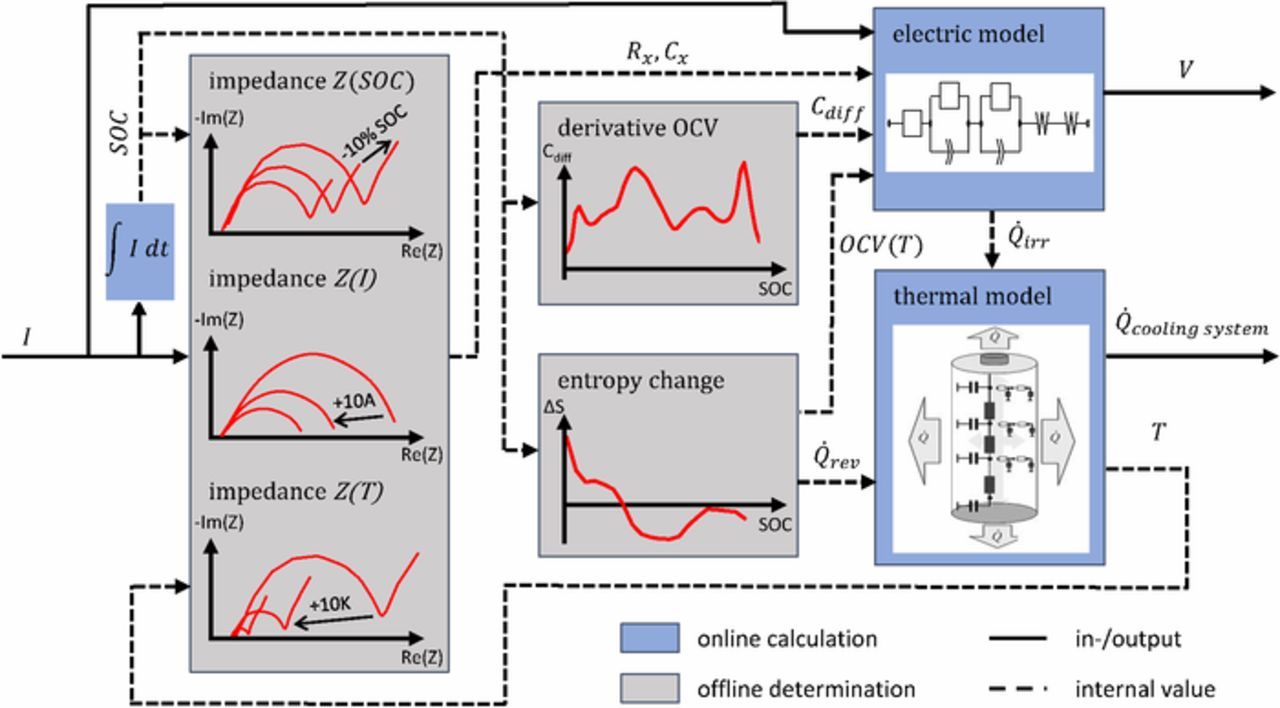
The ECM for cells shown in Figure 6b can easily be extended to a battery system model. In Figure 7, a common model approach for a battery system is presented. This battery model contains different approaches and is reduced to the essential components as done in Refs. 125,126,137 The battery model consists of the electrical ECM, the thermal model and the model parameter sets. Such models allow online monitoring and control of batteries; the behavior is usually reproduced by simulating the response of the electrical-thermal model to a given current. The output signals of the electrical model are the voltage and the irreversible heat generation. The model parameters required for this purpose are determined from offline measurements carried out in advance. The inputs of the thermal model are the irreversible heat generation and the reversible heat generation due to the entropy change as well as the heat transfer to the cooling system or environment. Thus, the temperature change can be calculated. Due to the interaction of thermal and electrical processes in the model, the battery behavior can be predicted under a wide range of operating conditions.
Figure 7. Typical battery system model to calculate the voltage response V and the heat generation  cooling system to a given current I. The blue boxes calculate the output and internal values online by the use of the offline determined measurement curves (gray boxes). The solid line are the in- and output parameters and the dashed line represents the internal values of the battery system model. The internal values are the state of charge SOC, the charge transfer resistance Rx and the double layer capacity Cx for different conditions, the derivative of the OCV Cdiff, the reversible and irreversible heat generation
cooling system to a given current I. The blue boxes calculate the output and internal values online by the use of the offline determined measurement curves (gray boxes). The solid line are the in- and output parameters and the dashed line represents the internal values of the battery system model. The internal values are the state of charge SOC, the charge transfer resistance Rx and the double layer capacity Cx for different conditions, the derivative of the OCV Cdiff, the reversible and irreversible heat generation  rev and
rev and  irr, the temperature T as well as the temperature dependent open circuit voltage OCV(T).
irr, the temperature T as well as the temperature dependent open circuit voltage OCV(T).
Data-driven Models
Data-driven modeling techniques use historical data, real-time data, or both138–141 for the purpose of monitoring, diagnosis, design, understanding of physical phenomena and operation. This section considers data-driven methods for the prediction or estimation of the state of Li-ion cells and batteries.
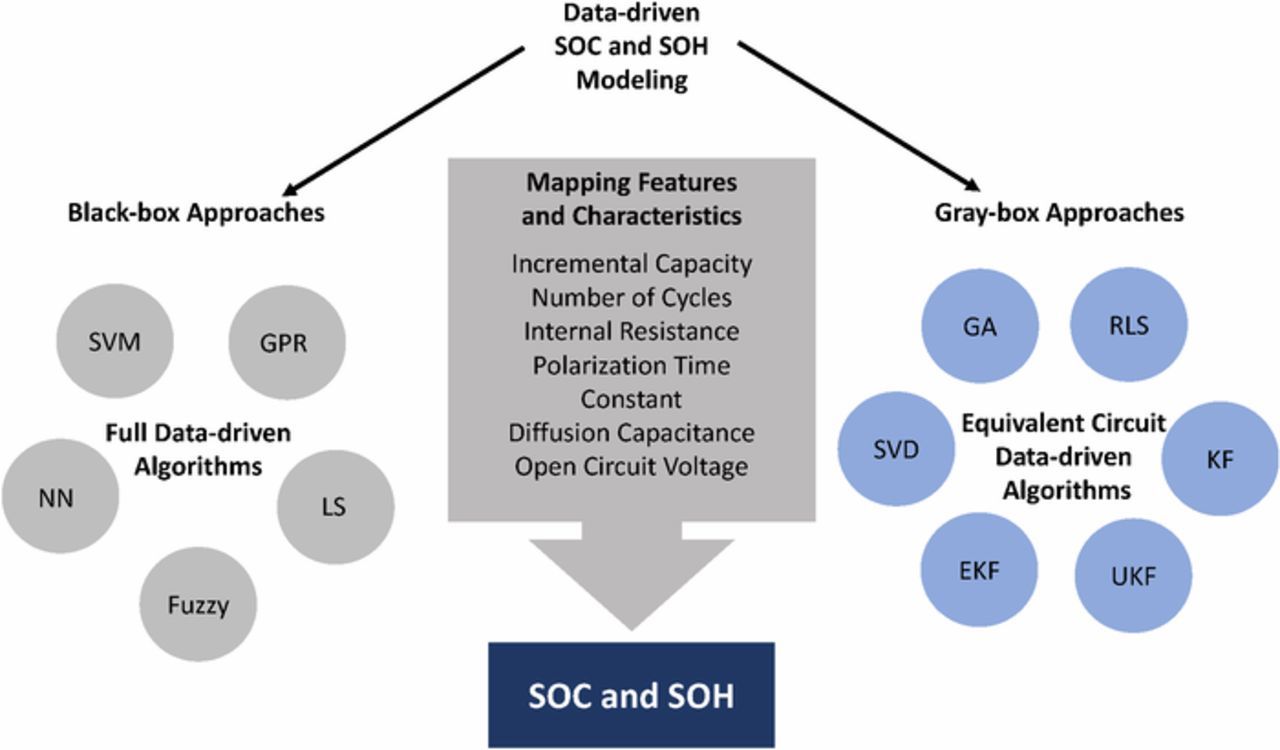
Figure 8 is a schematic on data-driven algorithms used to map the features of batteries commonly reported in the literature.
Figure 8. Popular data-driven algorithms, battery characteristics, and features for state-of-charge (SOC) estimation and state-of-health (SOH) prediction. Black-box approaches are completely based on data and no physically motivated model structure is assumed, while gray-box approaches are based on fusing a physically motivated model as well as data-driven techniques. The abbreviated terms for the techniques are support vector machine (SVM), Gaussian process regression (GPR), least squares (LS), neural network (NN), genetic algorithm (GA), recursive least square (RLS), Kalman filter (KL), unscented Kalman filter (UKF), extended Kalman filter (EKF), and singular value decomposition (SVD).
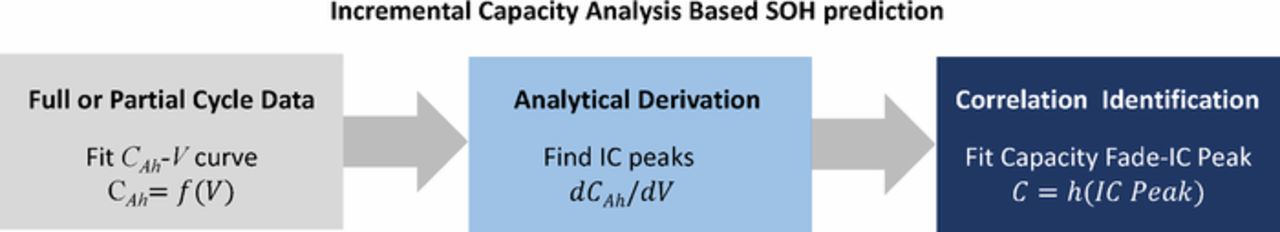
Most of the literature on data-driven methods is on the most important variables for the diagnosis and operation of batteries, SOC and SOH (see Introduction section). The main advantage of data-driven methods is that the underlying complicated physicochemical processes (see the discussion in Dynamic operation and changes in battery state and Dynamic processes and their time constants sections) that govern the SOC or SOH of Li-ion batteries do not need to be known. Instead, data-driven algorithms learn relationships between states and performance features empirically by using historical and/or real-time operational data. Some characteristics and features of Li-ion battery performance are vital to correlate to the SOC or SOH of the batteries. For example, the open circuit voltage (OCV) is a function of both SOC and SOH. Further, the peaks of incremental capacity (IC) curves, where IC = dCAh/dV is plotted vs. cell voltage V of batteries, reflect the aging of batteries, which in turn can be used to quantify the SOH of batteries.
The main objective of data-driven algorithms for Li-ion batteries is to learn the relationships between these measurable key performance features and the SOC or SOH, for use in the estimation of the SOC and prediction of the SOH.
This section broadly categories data-driven modeling techniques into two main groups: (1) black-box modeling and (2) gray-box modeling, where the data-driven modeling is coupled to another model type. Furthermore, data-driven modeling techniques can be subcategorized as online (aka on-board, on-the-fly, or real-time) and offline. Most online algorithms mainly utilize partially dynamic data while offline algorithms are mostly based on full-cycle data. As tracking and optimization of the state of batteries are essential for most practical applications, online algorithms are more relevant than offline algorithms for most practical applications. Online algorithms are especially relevant for applications in electric vehicles, hybrid electric vehicles, and plug-in hybrid electric vehicles.
The next two sections discuss black-box and gray-box modeling in SOC estimation and SOH prediction for Li-ion batteries, which has the been the main focus of the literature. Remarks and perspectives section discusses the application of these methods to other operational variables.
Black-box modeling
In black-box modeling, c.f. Figure 9, the relationships between battery features (or characteristics) and SOC or SOH are learned completely based on historical or operational data. In general, the underlying relationships are nonlinear, so nonlinear data-fitting algorithms are applied for SOC estimation and SOH prediction.
Figure 9. An example of black-box modeling: a basic framework of state-of-health (SOH) prediction by using the incremental capacity analysis (ICA) method.
Incremental capacity analysis is a common technique142 to analyze the capacity fade of Li-ion batteries. The capacity fade was mainly due to the SEI formation at the negative electrode (see the discussion in Dynamic operation and changes in battery state section). As SOH can be quantified via capacity fade, incremental capacity analysis is an effective tool to predict the SOH. The basic idea of using it for SOH monitoring is first to fit the charged capacity (CAh) vs. battery voltage using either full-cycle data or normal operational data. Then the identified model is used to determine the IC peaks analytically. As IC peaks are sensitive to capacity fade – IC peaks decrease as battery capacity fades – the next step is to fit a correlation between the highest sensitive IC peak and the amount of capacity fade. The identified IC peak-capacity correlation can then be used to predict the SOH of the battery. Figure 9 shows the basic concept of ICA-based SOH prediction for Li-ion batteries.
Machine learning algorithms such as support vector regression have been applied to identify the underlying nonlinear correlations between battery features and the SOH or SOC. The basic idea of support vector regression is to map the input space data via a nonlinear function into a feature space such that a linear regression can be formulated in the feature space. Support vector regression approaches attempt to achieve generalized predicting power by using sample data.143 A sparse algorithm is proposed in Ref. 144 to fit the CAh-V curve using electric operational data during charge. With the identified CAh-V model, the correlation between normalized capacity and normalized peaks are then identified. An advantage of the proposed technique is its suitability for online application that leverages normal operational data in vehicles. However, the proposed strategy does not consider the effects of ambient or cell temperature on the IC curve and peaks, which is one of the most significant degradation factors in automotive applications.138 Environmental temperature could be included as an additional input into any data-driven model, which would fit instantaneous effects but not the history of environmental temperature variations known to affect SOH, SOC, and battery lifetime.
An alternative data-driven approach is an artificial neural network algorithm145 that uses the ambient temperature, dynamic operational current, and voltage data to predict the SOH of Li-ion cells. The proposed technique first uses the K-means clustering algorithm, which is a popular unsupervised machine learning technique, to subgroup historical data of different driving patterns. The unsupervised algorithm uses the input data, i.e. temperature, voltage, and current, without labeled responses, i.e. different driving patterns. The identified subgroups are then used as inputs to an artificial neural network, which is then learned with historical data to predict the capacity. The learning process is performed offline. Once the online data are available, the data are first classified and are represented as densities in subregions. Here the densities of subregions refer to the number of points assigned to the subregions (for more details, see Ref. 145). Then the density vector is used to predict SOH of cells by using the learned neural network. Although the proposed technique is fast enough for on-line application, with less than 100 ms for one SOH prediction to be applied in online operation, the training data do not consider the resting of cells after each cycle. The resting in between cycles reflects a more practical scenario for Li-ion batteries and also exhibits a sudden increment in capacity (aka regeneration or self-recharge phenomenon) for the next cycle;146,147 for physical details see Dynamic processes and their time constants section. In Ref. 147 such a regeneration of capacity is taken into account when predicting the SOH of Li-ion batteries. The algorithm uses Gaussian process regression algorithms to predict the SOH by using a number of cycles. The main advantage of such techniques is their explicit accounting of the effects of uncertainty in their predictions. The proposed algorithm uses a sinusoidal covariance function to capture regeneration phenomena and a radial basis function, as another covariance function, to capture the general degradation. The mean function is either linear or quadratic. This modified Gaussian process regression algorithm is referred to as combination Gaussian process functional regression.147 Although it effectively captured the regeneration and the general degradation phenomena, temperature effects were not addressed. Furthermore, the training data do not reflect real dynamic operations and so potential application of the proposed technique is limited.
Besides SOH estimation, the black-box modeling approach also has been applied to SOC estimation. In Ref. 148, a fuzzy logic SOC estimator was designed and implemented for Li-ion batteries. The SOC estimator was designed in particular for the application in automated external defibrillators, where the load profile consists of pulses. The SOC was characterized in terms of the number of current pulses that a battery can deliver at a given time. Thus, the number of load pulses that a battery can deliver reflects the capacity in this particular application. The fuzzy logic algorithm used features of voltage recovery and minimum and maximum difference voltages during the current loading of battery packs as inputs to the model. Using experimental data, a Sugeno-type fuzzy inference system was trained to learn the correlation between the voltage features and the number of pulses remaining. Although an fuzzy logic estimator of SOC had high accuracy (one pulse error), the fuzzy inference system did not consider temperature effects in its SOC estimation. In Ref. 149 the effects of temperature was considered when developing a fuzzy logic estimator of SOC. The proposed technique used classical EIS measurements (see Dynamic measurement methods section) to infer the SOC. A practical limitation of EIS is the need for batteries or cells to operate at steady state.141
One reason that the literature on black-box methods focuses on SOC estimation and SOH prediction is that the methods are limited to the measurements that are available. The models are not faster to apply than ECMs, which can describe the local dynamics needed for control purposes, so the motivation for using black-box methods for battery control is limited. Information on battery degradation mechanisms or any other physicochemical information are not needed by black-box methods, which can be argued to be an advantage; however, black-box methods also cannot exploit such information and so are limited in terms of the achievable performance for SOC estimation, SOH prediction, and optimizing operations and control. Another limitation of black-box models is that the model parameters are not relatable to any parameters of the system. As such, the quantification of uncertainties in model parameter estimates is not as useful as for the P2D model,150 in which such analyzes can track and quantify the degradation of specific components by tracking with confidence the variation in the physical model parameters (see also Single-cell models with homogeneous electrodes section).
The computational cost of constructing a black-box model for most data-driven methods (including machine learning and artificial neural networks) is orders of magnitude higher than for ECMs but is lower than the most sophisticated first-principles models. Once a black-box model is constructed, the cost of running the black-box model in parallel with operations online is low. The high computational cost of construction of many black-box mode remains a drawback online since the black-box model needs to be reconstructed as the battery ages. One approach could be reconstruct the black-box model only occasionally, when significant change in the battery behavior has been detected.
Although the effectiveness of black-box models depends on the battery chemistry and the shape of the discharge curve, by being based only on data, the steps in their application is the same. In contrast, mechanistic models need to be modified when changing to battery chemistries in which different phenomena occur, such as moving from a battery with single to multiple solid phases (e.g., Ref. 92).
Fusing data-driven modeling with other model types: grey box modeling
Black-box modeling requires a significant amount of data for correlating variables, while discarding any other information that would decrease the amount of data for training and estimation. In gray-box modeling, the data-driven approach is combined with other model types, by fusing the complementary information derived from multiple approach. In the literature, equivalent circuits have been overwhelmingly utilized to model lumped phenomena such as charge transfer and diffusion that occur in Li-ion cells.138,151,152 As discussed in Equivalent circuit and impedance models section, these phenomena can be represented by using parallel RC circuits, which model the dynamic response of a battery voltage. In this section, RC-equivalent circuit-based data-driven approaches are discussed. As the focus is on SOC estimation and SOH prediction, as that has been the focus of the literature in this area.
The main idea of combined RC-equivalent circuit and data-driven modeling approaches is shown in Fig. 10. In the SOC estimation algorithms used in this framework, most data-driven approaches map the correlation between the OCV and SOC. Note that an identified OCV-SOC model could be directly used to determine the SOC of batteries after resting a certain time to reach steady state after operation of the battery. A long resting period is not practical,139 however, and the transient behavior of the battery must be taken into account when determining the SOC; here, equivalent circuits allow the deduction of open circuit voltage from dynamic battery voltage and current. The values of RC-equivalent circuit parameters also reflect the SOH of a battery. Therefore, in RC-equivalent circuit algorithms for SOH prediction, most data-driven approaches mainly focus on mapping variations in lumped parameters from equivalent circuits to the SOH of cells or batteries. The gray-box modeling framework follows two main steps: first equivalent circuit parameters are identified by using online operational data or offline experimental data, and then by employing both equivalent circuit and data-driven modeling, the SOH is predicted by using the identified parameters, and the SOC is estimated using the equivalent circuit model with the identified parameters.
Figure 10. The basic idea of RC equivalent circuit-based gray-box modeling for estimating SOC and predicting SOH of batteries. The f, g, h, l refer to analytical expressions.
In Ref. 153 the OCV-SOC nonlinear relationship was characterized using sigmoid functions. The sigmoid functions are shown to effectively capture the staging phenomena during intercalation and deintercalation of lithium, compared to other nonlinear functions used in the literature. By using the overall model, an extended Kalman filter (EKF) algorithm was then proposed to estimate the SOC of the battery. The application of the proposed technique is, however, limited as the method used constant RC parameters in the equivalent circuit. The RC parameters are time varying due to aging or temperature, and hence need to be identified online using operational data to be able to accurately model the battery. In Ref. 152 the OCV-SOC relationship was mapped offline with two polynomial functions, one for charging and one for discharging. The RC parameters were then identified for both charging and discharging processes using a genetic algorithm. A robust EKF algorithm was proposed to estimate the SOC of batteries in order to cope with uncertainties in the identified RC model parameters and measurement noise. Although the robust EKF estimator had a better convergence of the SOC to the true value compared to an EKF algorithm, the proposed robust EKF design did not explicitly consider uncertainties in parameters or the initial SOC. Uncertainty in the initial SOC is one of the main challenges for estimating SOC using direct methods139 such as coulomb counting, open-loop, or Ampere-hour integral algorithms.
In the literature, alternatives to direct SOC algorithms are referred to as indirect estimation algorithms (such as the EKF118,119,154). These indirect algorithms should explicitly take into account the initial SOC uncertainty to be comparative with direct algorithms. In general, EKF algorithms may diverge (mean and variance) due to the coarse approximation associated with linearizing of the system dynamics (first-order accuracy) when propagating through the nonlinear systems. An alternative to EKF algorithms are unscented Kalman filter algorithms, which can handle nonlinearity effectively with statistics that are accurate up to third order.155 They have been applied for SOC estimation.41 Further, particle filter algorithms, which can effectively tackle both nonlinearity and non-Gaussian random variables, have also been applied to the estimation of SOC in batteries.44,156 However, particle filter algorithms have a slow rate of convergence.156
An alternative to such state estimation algorithms is an online algorithm157 in which the OCV is identified as a parameter along with other RC parameters in the identification step. The identification can be performed online, i.e. by evaluating current and voltage during operation, using a recursive least squares algorithm. The identified OCV is then used to determine the corresponding SOC by using an OCV-SOC lookup table, which has been formulated offline. Although the technique is a promising online algorithm, storing the OCV-SOC lookup table may be expensive, as the size of the memory requirements grow with the number of points in the OCV-SOC table, which in turn determines the resolution of the SOC. To reduce the memory requirements, data reduction techniques such as singular value decomposition can be applied for the OCV-SOC relationship.158,159 In general, lookup table approaches may need to apply interpolation techniques to determine the SOC, which is a drawback compared to analytical OCV-SOC expression-based estimation techniques.
Besides SOC estimation, the RC-equivalent circuit-based gray-box modeling framework has been applied to predict the SOH of batteries. A degradation index is defined in Ref. 160 using the internal resistance of a battery's RC-equivalent circuit model, and it is shown to reflect the SOH of batteries. The proposed algorithm first identified online the internal resistance of the RC model using a least squares algorithm. Then, the SOH was predicted using a lookup table in which the internal resistance over temperature data of the new cell had been stored. The predictive technique had low enough computational effort to be suitable for practical electric vehicle applications. The accuracy of the prediction depends on the number of grid points used in the lookup table. Storing a large amount of data points may be expensive as discussed before and use of interpolation techniques may cause less accurate predictions. In contrast, the SOH is directly parameterized in Ref. 161 in terms of diffusion capacitance and temperature, and the capacity of a battery is shown to increase as temperature increases and decrease as diffusion capacitance increases. The diffusion capacitance reflects the effect of concentration dynamics and thus voltage dynamics due to slow Li diffusion, represented in equivalent circuits by an additional diffusion-related RC-circuit. The technique first identified the diffusion capacitance online using a genetic algorithm and then predicted the SOH of the battery using the parameterized model. The proposed technique requires fast identification of OCV as a parameter (to avoid OCV changes significantly during the identification step). While the proposed technique has a better SOH prediction capability ( ∼ 5% error in SOH), a drawback noted in Ref. 161 is that the GA does not converge quickly.
Remarks and perspectives
The prediction of remaining useful life is an other important task of battery management systems. In the literature, remaining useful life is defined as the number of cycles remaining until a battery reaches its end of life. The latter is usually considered as 80% of rated capacity or 80% of SOH. Numerous articles have been published on data-driven methods for RUL estimation, including artificial neural networks,162 support vector machine,163 and relevance vector machine164 algorithms, and the reader is referred to these papers and references therein for more details. As the remaining useful life is a function of the current cycle and the SOH of a battery, common ideas of these algorithms are first to estimate the current cycle number162,163 or SOH164 of a battery and then to project the remaining useful life using current cycle number-remaining useful life or SOH-remaining useful life relationships, respectively. This section focuses on data-driven and on coupled equivalent circuit-based data-driven techniques for the SOC estimation and SOH prediction, primarily with a focus on guidance on when to use which methods in which application. In Ref. 165 Gaussian process modeling has been used to predict the battery state of health and the remaining usful life of a battery.
As discussed before, the main objective of data-driven approaches for SOC estimation and SOH prediction is to map the underlying nonlinear relationships between battery characteristics or features, and the SOC or SOH. Such correlations need to be time varying mainly due to aging and environmental conditions, e.g. temperature. Online algorithms that learn the nonlinear correlations using operational data are promising, especially in electric vehicle applications. However, the current data-driven literature tends to follow sequential approaches, for example, common SOC estimation techniques such as extended Kalman filter first identify the parameters and then estimate SOC. Instead, a promising route would be to use a dual-estimation framework in which parameters and the SOC are estimated simultaneously. Future research should also consider developing robust or stochastic estimation and prediction techniques that take explicitly into account measurement noise and uncertainties in parameters and initial conditions of batteries.
Conclusions
A huge variety of Li-ion battery models are available which are largely tailored for specific tasks. Selecting the most suitable model for a given purpose in terms of required accuracy, insight into the battery, simulation time and training or parameterization is challenging and requires a sound overview of their pros and cons. This article presented the available options and open challenges when using dynamic battery models for diagnosis, as well as operation and control of Li-ion batteries. Diagnosis and control of batteries needs to take into account the processes that determine the safety and performance of the cells, and thus it requires an understanding of the main processes, parameters, and time constants. Li-ion batteries are multiscale in nature, that is, they have time scales that span many orders of magnitude and these processes interact with each other. We presented a systematic overview on the range of time constants expected for the single processes in the battery and how to calculate these time constants. Together with an overview on dynamic measurement methods, the value of such methods for assessing dynamic behavior was discussed. Dynamic measurements are not limited to understanding battery behavior, but are essential also for model parameterization and state estimation. Here, a combination of various methods, including nonlinear methods, is crucial to establish uniqueness of the result.
We discussed three separate, yet closely connected model classes. Mechanistic models provide deep insight into the physical and chemical processes and state of the battery but are can be difficult to parameterize and computationally demanding, especially when containing detailed physical descriptions. Significant extensions are needed especially in the area of degradation modeling to enable accurate SOH estimation, and in the model-based understanding of multiscale effects and production influences including their uncertainties. Equivalent circuit models map the battery to a network of electric circuit components; ECMs can easily reproduce measured behavior but are limited in the depth of physical interpretation. Given the low on-line costs of ECMs, a future direction is to how to efficiently construct ECMs from more complicated first-principles models. The first-principles model would be operating only occasionally while the ECM would be operating in real time. Finally, the literature on data-driven models is discussed. Data-driven models are typically constructed using least-squares, machine learning, or artificial intelligence approaches. Although in principle data-driven models may cover complex behavior such as aging, their application is limited by the difficulty in incorporating any known details on first-principles mechanism. Also, data-driven model need to be trained to every situation. Some of the limitations are reduced by coupling data-driven modeling with equivalent circuit models. An open question is how to best tailor these methods to include some semi-quantitative information on the battery operation, e.g., how to account for battery chemistries that have multiple stable solid phases. Each of the model classes has a range of model variants which allows to select the best model variant for a given purpose.
In the authors' opinion, diagnosis and optimal operation and design of batteries require models that are able to span multiple time scales and allow physical insight and ease of parameterization. None of the existing modeling approaches is sufficiently versatile yet simple to bridge this gap. Thus, one of the biggest challenges is a fusion of the existing modeling approaches, fully exploiting their potentials and integrating first-principles physical insight and measurement data.
Acknowledgment
The authors acknowledge financial support from the MIT Germany Seed Fund.
ORCID
Ulrike Krewer 0000-0002-5984-5935
Richard D. Braatz 0000-0003-4304-3484
Rolf Findeisen 0000-0002-9112-5946