Abstract
In this study the PtNi/Nifoam catalyst was prepared by a simple approach. A thin Ni layer was deposited on the Nifoam substrate, using sodium hypophosphite as a reducing agent. Then, Pt crystallites were deposited on Ni/Nifoam by its immersion into 1 mM H2PtCl6 + 0.1 M HCl at 25 °C for various time periods. FESEM, EDX, and ICP-OES were used for the examination of the morphology and composition of catalysts. The catalytic activity of the prepared PtNi/Nifoam was evaluated toward the hydrolysis of NaBH4 and compared with that of Ni/Nifoam. It has been determined that the PtNi/Nifoam catalysts have higher catalytic activity with respect to the generation of hydrogen as compared to Ni/Nifoam.
Export citation and abstract BibTeX RIS
NaBH4 (sodium borohydride) is of interest as a source for a hydrogen generation during its catalytic hydrolysis,1 due to its nontoxicity, high stability in strongly alkaline media under ambient operation conditions and easily controllable hydrogen generation (HG) rate:1,2
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/164/2/D53/revision1/d0001.gif)
The rate of HG significantly depends on a suitable catalyst applied as well as on its structure or support selected. Various compositions of non-noble or noble metals catalysts such as Co or Ni based catalysts,3–10 several ternary catalysts Co-X-B (X = Ni, P, Cr, Fe, Cu, Mo, W),4,11–19 Pt or Pt-based catalysts,20–30 Ru31–34 and Rh35 have been used for catalytic hydrolysis of sodium borohydride. The search of catalysts with high activity toward the hydrolysis reaction of sodium borohydride and durability plays an important role in the hydrogen generation for fuel cells. The supports having an increased surface area or dopants which provide good dispersion of the catalyst and sufficiently enlarge the contact area with the reactants are commonly used with the aim to increase the activity of heterogeneous catalysts. The catalysts having different nanostructured morphologies have been developed. Among them, Co films composed of mesoporous Co-B nanocatalysts with slit-like pores of different size,36 hollow spheres,37 honeycomb-like38 and flower-like,39 mesoporous structures of Co-B,40 copper-cobalt foams41 have been synthesized to improve the hydrolysis of NaBH4.
In this study a simple approach for fabrication of catalysts for hydrogen generation from NaBH4 by chemical methods using Nifoam as a substrate is presented. Field emission scanning electron microscopy, energy dispersive X-ray spectroscopy and inductively coupled plasma optical emission spectroscopy were used for the examination of the morphology and composition of catalysts. The catalytic activity of prepared PtNi/Nifoam was evaluated toward the hydrolysis of NaBH4 and compared with that of Ni/Nifoam.
Experimental
Formation of catalysts
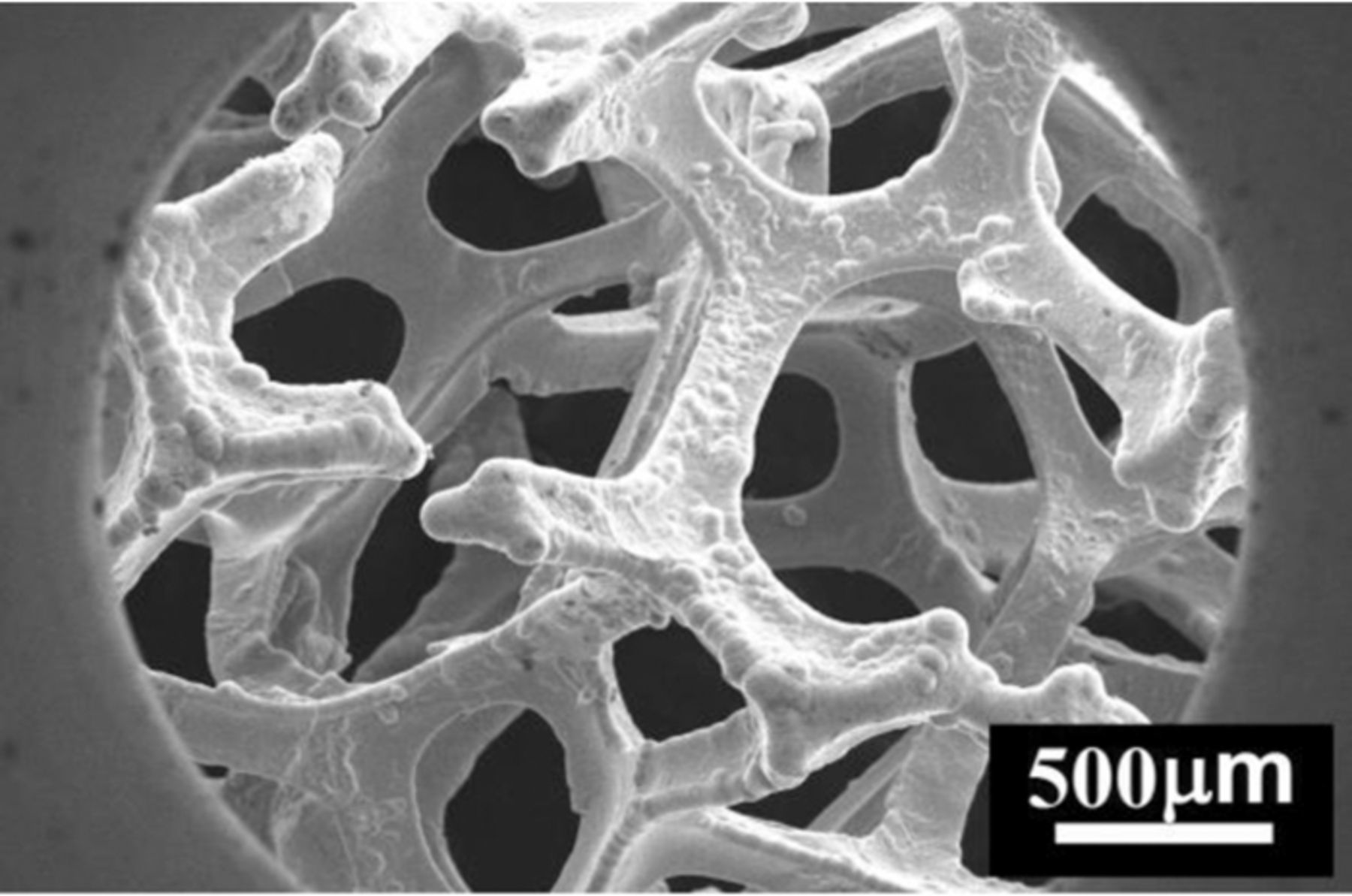
The Nifoam with the number of pores per inch (PPI) of 30–40, the density of 900 g m−2 and the thickness of 3.5 mm was purchased from Zhenjiang Global Industrial Components Trading Co., Ltd. (China) Supplier. Figure 1 shows a SEM view of Nifoam as-purchased.
Figure 1. SEM view of Nifoam as-purchased.
For the fabrication of catalysts Nifoam sheets of 1 cm × 1 cm were used. At first, a thin Ni layer was electrolessly deposited on the surface of Nifoam, using sodium hypophosphite as a reducing agent as described in detail in Ref. 42. The surface of Nifoam, activated with Pd, was immersed in the nickel plating solution for 1 min at a temperature of 85 °C.
Pt crystallites were deposited on the prepared Ni/Nifoam surface via galvanic displacement. The Ni/Nifoam surface was immersed into the 1 mM H2PtCl6 + 0.1 M HCl solution at a temperature of 25 °C for 5 and 15 min, respectively.
Characterization of catalysts
A SEM/FIB workstation Helios Nanolab 650 with an energy dispersive X-ray (EDX) spectrometer INCA Energy 350 X-Max 20 was used for the examination of the morphology and composition of the fabricated catalysts.
The Pt metal loading in the PtNi/Nifoam catalysts was estimated from ICP-OES measurements by recording ICP optical emission spectra using an ICP optical emission spectrometer Optima 7000DV (Perkin Elmer).
Hydrogen generation measurements
The amount of generated hydrogen was measured by using a MilliGascounter (Ritter). Briefly, the reaction solution containing 5 wt% NaBH4 and 0.4 wt% NaOH (v = 15 ml) was thermostated in an airtight flask, which was fitted with an outlet for collection of evolved H2 gas, connected with the MilliGascounter. Then the prepared Ni/Nifoam or PtNi/Nifoam catalysts were added into the solution maintained at the designated temperature to initiate the hydrolysis reaction of sodium borohydride. The hydrogen generation rate was measured at different solution temperatures (50, 60 and 70 °C) in order to determine the activation energy.
Results and Discussion
In this study, the PtNi/Nifoam catalysts were prepared by a simple and cost-effective approach. At first, a thin Ni layer was deposited on the surface of Nifoam, using sodium hypophosphite as a reducing agent. Figure 2 presents SEM views of Nifoam before (a) and after electroless Ni plating (b). As seen from the data of SEM analysis in Fig. 2b, the electrolessly deposited Ni on the Nifoam surface produces a layer of granular nickel particles up to 200 nanometers in size. As sodium hypophosphite was used as a reducing agent for the deposition of Ni sublayer on Nifoam, phosphorus was detected in the Ni coating as evident from the data in Table It2. The amount of Ni, deposited on the Nifoam surface, was determined gravimetrically and is ca. 300 μg.
Figure 2. SEM views of Nifoam before (a) and after electroless Ni plating (b).
Table I. Composition of the Ni layer deposited on the Nifoam surface determined by EDX.
| Elements, at% | |||
|---|---|---|---|
| Catalyst | Ni | P | Pd |
| Ni/Nifoam | 90.61 | 9.11 | 0.28 |
Table II. Composition of the PtNi/Nifoam catalysts determined by EDX. The Pt loading in the catalysts was determined by ICP-OES.
| Elements, at% | ||||||
|---|---|---|---|---|---|---|
| Catalyst | Pt | Ni | P | Pd | tPtdep, min | MPt, μg |
| PtNi/Nifoam | 0.85 | 85.07 | 12.61 | 1.47 | 5 | 121 |
| 1.54 | 81.17 | 16.83 | 0.46 | 15 | 158 | |
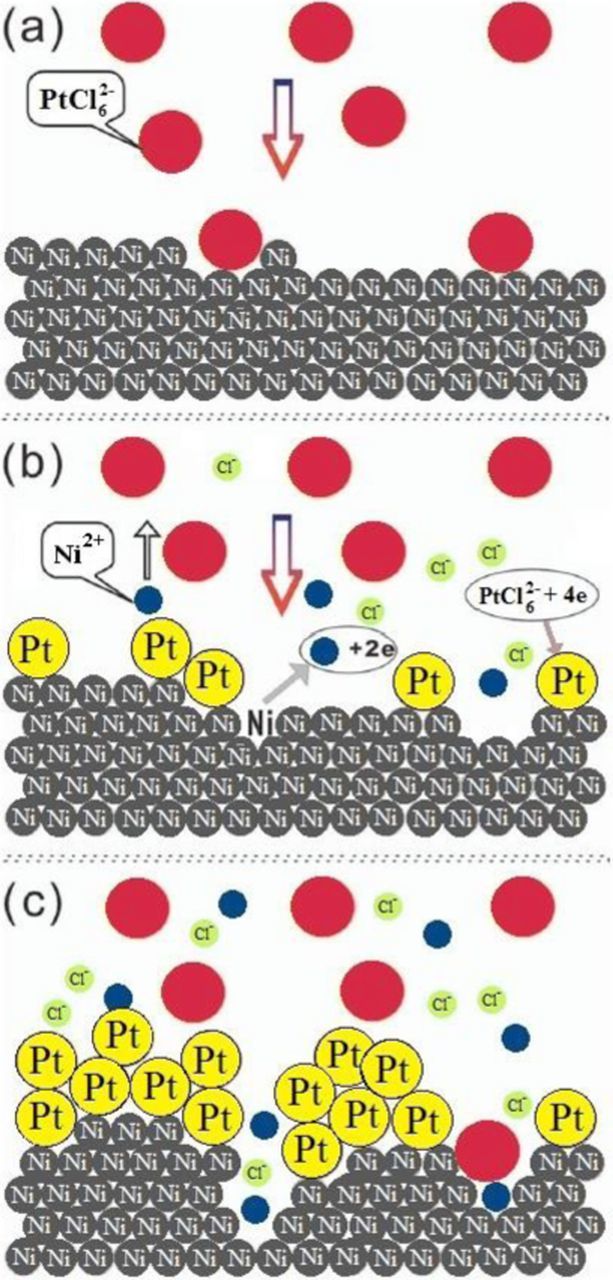
Pt crystallites were deposited on the Ni/Nifoam surface by immersion of the Ni/Nifoam electrodes in the H2PtCl6 + 0.1 M HCl solution at a temperature of 25 °C for 5 and 15 min, respectively. This simple procedure is known as galvanic displacement or immersion plating, during which the deposition of a noble metal occurs by the oxidation of a precursor metal adlayer deposited on the substrate at the open-circuit potential. Figure 3 shows the scheme of Pt deposition on the Ni surface through galvanic displacement. At first, PtCl62− diffuses from the solution bulk to the surface of Ni, then it is adsorbed on the Ni surface (Fig. 3a). Subsequently, Ni atoms are oxidized and dissolved into the solution, whereas Pt4+ from the adsorbed PtCl62− is simultaneously reduced to the metallic state on the surface of Ni (Eq. 2) (Fig. 3b):
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/164/2/D53/revision1/d0002.gif)
Figure 3. The scheme representing different stages of the process of galvanic displacement of Ni by Pt.
In addition to that, when the Ni surface is basically covered with Pt deposit, it can be oxidized and dissolved through tiny holes on the Pt deposit (Fig. 3c).
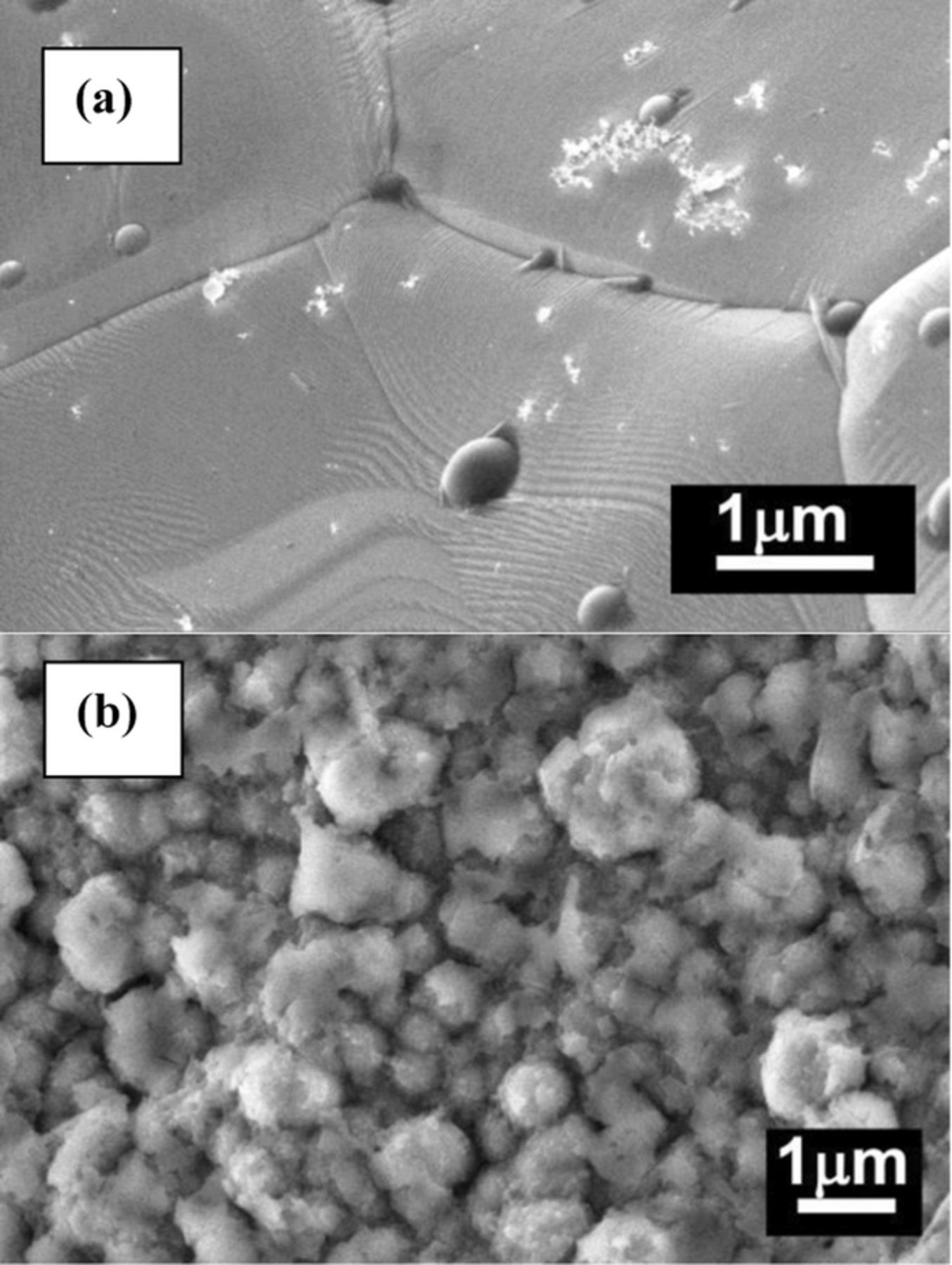
SEM views of the PtNi/Nifoam catalysts are shown in Fig. 4. As seen, the immersion of the prepared Ni/Nifoam electrode into the 1 mM H2PtCl6 + 0.1 M HCl solution for 5 min results in the deposition of light oblong sticks of platinum sized 10 to 200 nanometers and formation of a particular structure of flowerlike nanorods aggregates on the catalyst surface (Fig. 4a). When the Pt deposition time was 15 min, Pt crystallites agglomerated and increased (Fig. 4b). The EDX analysis of different PtNi/Nifoam catalysts confirms the presence of Pt and Ni (Table II). The amount of deposited Pt in the catalysts was determined by ICP-OES and was about 121 and 160 μg, respectively.
Figure 4. SEM views of PtNi/Nifoam prepared by immersion of Ni/Nifoam in H2PtCl6 + 0.1 M HCl at 25 °C for 5 (a) and 15 (b) min.
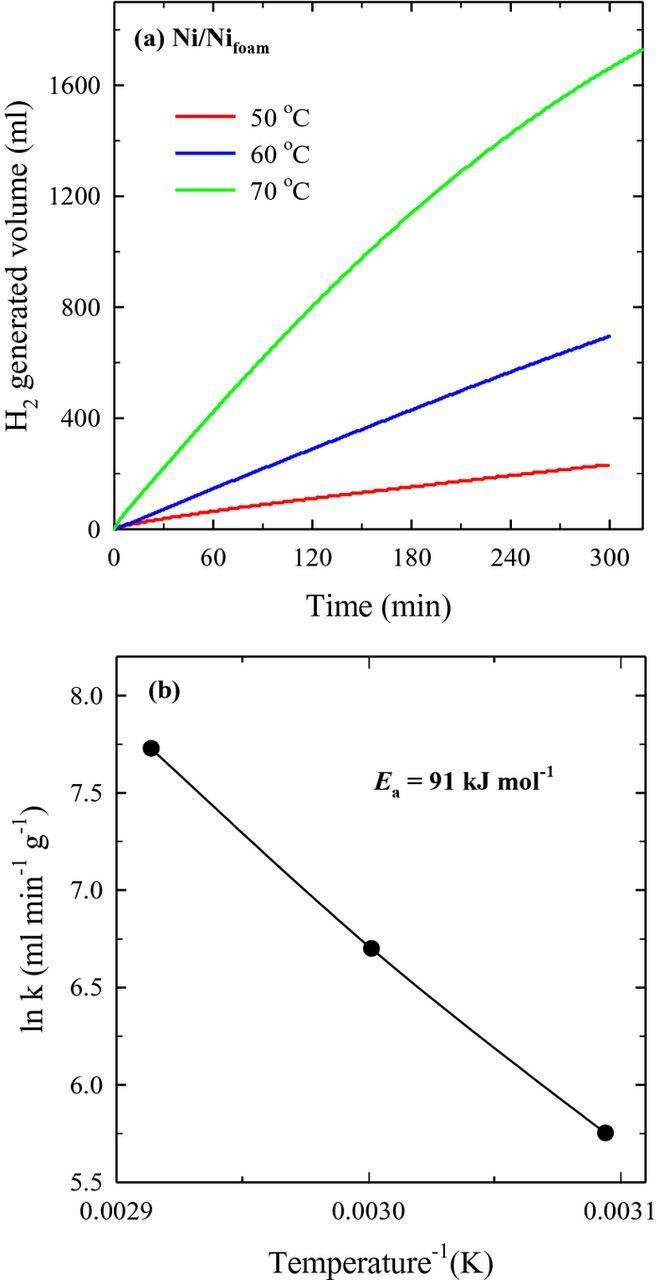
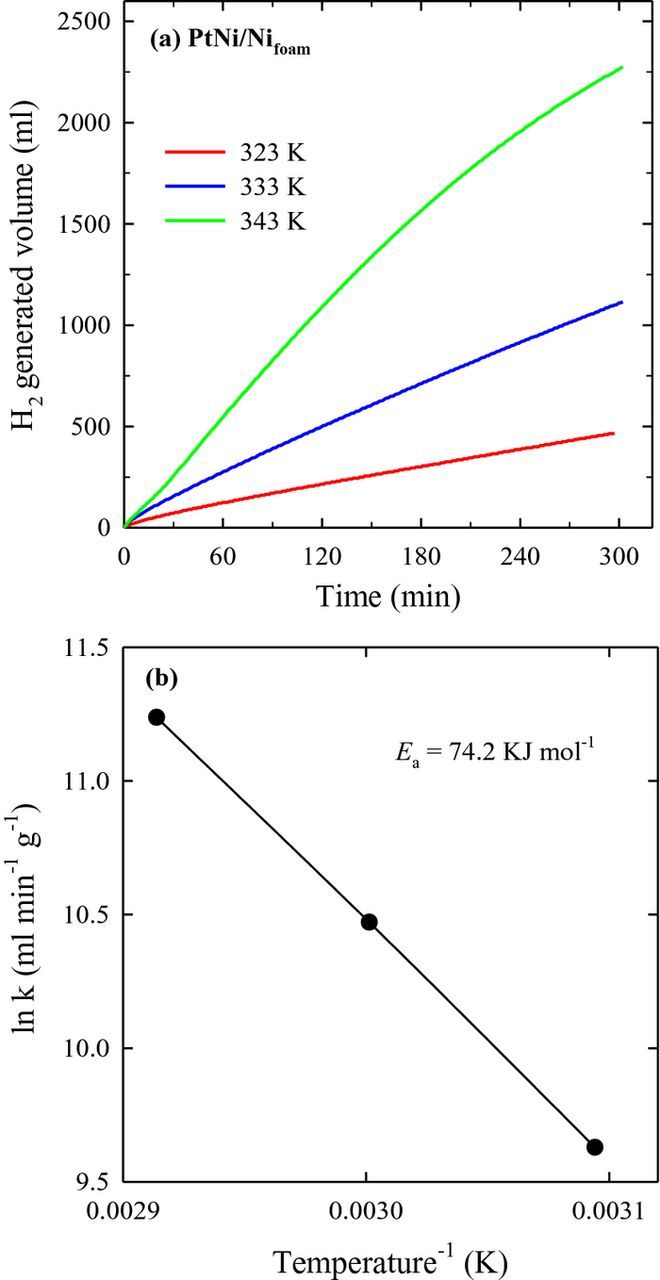
Hydrolysis of NaBH4 was investigated using the prepared catalysts at various temperatures. Figures 5 and 6 present the hydrogen generation rate measured on the Ni/Nifoam and PtNi/Nifoam catalysts with the Pt amount of 121 μg at temperatures of 50–70 °C. The rate of catalytic hydrolysis of NaBH4 in an alkaline solution increases exponentially with increase in reaction temperature, as evident from Figs. 5 and 6. Data on hydrogen generation rates on the Ni/Nifoam and different PtNi/Nifoam catalysts are given in Table III. As seen, the PtNi/Nifoam catalysts outperformed Ni/Nifoam. Deposition of a low amount of Pt on Ni/Nifoam significantly increases hydrogen generation rates from the sodium borohydride solution.
Figure 5. (a) H2 generation from 15 ml 5 wt% NaBH4 + 0.4 wt% NaOH catalyzed by Ni/Nifoam at different temperatures. (b) The Arrhenius plot calculated from the rates of NaBH4 hydrolysis in the same solution.
Figure 6. (a) H2 generation from 15 ml 5 wt% NaBH4 + 0.4 wt% NaOH catalyzed by the PtNi/Nifoam catalyst with the Pt amount of 121 μg at different temperatures. (b) The Arrhenius plot calculated from the rates of NaBH4 hydrolysis in the same solution.
Table III. Hydrogen generation rate obtained from 15 ml 5 w % NaBH4 + 0.4 w % NaOH catalyzed by the Ni/Nifoam and PtNi/Nifoam catalysts.
| Ni/Nifoam catalyst | PtNi/Nifoam catalyst | |||
|---|---|---|---|---|
| Temperature, °C | Ni loading, μg | H2 generation rate, ml min−1 gNi−1 | Pt loading, μg | H2 generation rate, ml min−1 gPt−1 |
| 50 | 300 | 313.99 | 121 | 15150.19 |
| 60 | 809.51 | 35177.84 | ||
| 70 | 2262.48 | 75690.66 | ||
| 60 | — | — | 158 | 11430.87 |
The highest hydrogen generation rate was obtained at PtNi/Nifoam with the Pt amount of 121 μg at a temperature of 70 °C and is ca. 76 L min−1 gPt−1. The hydrogen generation rate at 60 °C is ca. 5 and 43 times higher on PtNi/Nifoam with the Pt amounts of 158 and 121 μg, respectively, as compared to that on bare Ni/Nifoam.
The Arrhenius plot was calculated from the rates of NaBH4 hydrolysis for Ni/Nifoam and PtNi/Nifoam with the Pt loading of 121 μg and is shown in Figs. 5b and 6b, respectively. The Arrhenius plot gives the activation energy of 91 kJ mol−1 for Ni/Nifoam and 74.2 kJ mol−1 for PtNi/Nifoam.
It should be noted that the PtNi/Nifoam catalyst, prepared by immersion of Ni/Nifoam into the platinum-containing solution for 15 min, shows slower kinetics of borohydride hydrolysis as compared to that prepared by immersion of Ni/Nifoam into the platinum-containing solution for 5 min. Deposition of light oblong sticks of Pt sized 10 to 200 nanometers with the particular structure of the flowerlike nanorods aggregates on the catalyst surface, as evident from the SEM data in Figure 4a, results in enhanced hydrogen generation rates.
Comparison of hydrogen evolution on catalysts prepared in this work with that obtained on various other catalysts reported in the literature is summarized in Table IV. It is evident that the catalytic activity of the prepared PtNi/Nifoam catalyst with the amount of Pt equal to 121 μg is higher as compared with those of the noble metal catalysts such as Pt/AC, Ru/IR-120, Ru/C20,27,28 and noble metal-based catalysts: Pt/Si3N4, CoPt-PEDOT:PSS/MWCNT and Ni-Ru/50WX8.24,26,34
Table IV. Comparison of hydrogen generation (HG) on various catalysts.
| Catalyst sample | T, °C | Composition of solution | HG | Ref. |
|---|---|---|---|---|
| Pt/AC | 30 | 5 wt% NaBH4 + 5 wt% NaOH | 8.5 L min−1 gPt−1 | 20 |
| Ru/IR-120 | 25 | 5 wt% NaBH4 + 1 wt% NaOH | 13.2 L min−1 g−1 | 27 |
| Ru/C | 25 | 1 wt% NaBH4 + 3.75 wt% NaOH | 12.9 L min−1 g−1 | 28 |
| CoPt-PEDOT:PSS/MWCNT | 25 | 150 mM NaBH4 + 0.4 wt% NaOH | 6.9 L min−1 g−1 | 26 |
| Ni-Ru/50WX8 | 35 | 10 wt% NaBH4 + 5 wt% NaOH | 0.4 L min−1 g−1 | 34 |
| Pt/Si3N4 | 80 | 120 mg NaBH4 + 2% NaOH | 24.2 L min−1 gPt−1 | 24 |
| PtNi/Nifoam | 50 | 5 wt% NaBH4 + 0.4 wt% NaOH | 15.1 L min−1 gPt−1 | This study |
| PtNi/Nifoam | 70 | 5 wt% NaBH4 + 0.4 wt% NaOH | 75.7 L min−1 gPt−1 | This study |
Conclusions
A simple approach to fabricate the PtNi/Nifoam catalyst has been presented. PtNi/Nifoam catalyst was prepared in two steps: electroless deposition of nickel onto the Nifoam surface, followed by deposition of Pt crystallites on Ni/Nifoam by its immersion into a 1 mM H2PtCl6 + 0.1 M HCl solution for various time periods.
Deposition of a low amount of Pt on Ni/Nifoam significantly increases hydrogen generation rates from sodium borohydride solution. The highest hydrogen generation rate was obtained at PtNi/Nifoam at 70 °C and is ca. 76 L min−1 gPt−1.
The fabricated catalysts seem to be a promising material for hydrogen generation from sodium borohydride hydrolysis. The catalysts obtained could have practical application in H2/O2 fuel cells as catalytic materials generating hydrogen from the sodium borohydride solution as fuel.
Acknowledgment
This research was funded by a grant (No. TEC-06/2015) from the Research Council of Lithuania.