Abstract
A novel composite anode is prepared by mixing zinc particles with activated carbon (AC) to improve the cycle performance of the neutral rechargeable zinc ion batteries. Galvanostatic charge/discharge cycling tests indicate that the capacity retention of the cell with adding 12 wt% activated carbon in Zn anode is 85.6% after 80 cycles, which is much higher than that of 56.7% for the cell using unmodified Zn anode. X-ray diffraction analysis indicates that the addition of activated carbon can suppress the formation of inactive basic zinc sulfates (Zn4SO4(OH)6·nH20). Morphology, elemental mapping and N2 adsorption and desorption measurements indicate that the pores of activated carbon can accommodate the deposition of Zn dendrites and insoluble anodic products. As a result, the cycle stability of the Zn anode has been greatly enhanced by activated carbon modification.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
Compared with other widely used metal anodes, such as lead, cadmium and rare metal mesh, zinc is a non-toxic, abundant, and low cost resource. Since the middle of 19th century, zinc has been used as an excellent anodic material for Zn/Mn, Zn/Ni and Zn/Ag batteries.1 With features such as low cost, environmental benignity and good specific energy, the Zn/MnO2 system has traditionally provided one of the most popular primary cells for a great number of applications. These attractive characteristics, combined with the growing need of high-performance batteries, have prompted considerable efforts to change its primary nature into a secondary one.
In the early 1980s, a rechargeable alkaline Zn/MnO2 (RAM) has been developed.1–4 Over the past three decades, the improvement of this technology has progressed rapidly. However, even now the rechargeability of batteries based on Zn anode in alkaline media remains a significant challenge. It is well known that the capacity of rechargeable alkaline Zn/MnO2 batteries using both KOH and LiOH electrolytes will dramatically drop to below 50% or even worse at only 40 cycles, meanwhile the columbic efficiency decreases to below 50%.2,4 It has been pointed out by Y. Shen and K. Kordesch4 that the capacity fading and columbic efficiency loss of rechargeable alkaline Zn/MnO2 batteries are mainly attributed to the formation of dendrites and passivation products in Zn anodes. These inactive dendrites or passivation products block the transfer of ions and slow the ion diffusion process, leading to a pronounced anodic capacity fading.4–10 In addition, the dendrites can penetrate into the separator and result in an interior short circuit. In the 1990's, the neutral ZnSO4 solution has been exploited as a replacement for the alkaline electrolyte with some promising results.11–13 Kim et al.13 reported that addition of 0.1∼0.5 mol L−1 MnSO4 to 2 mol L−1 ZnSO4 electrolyte greatly improved the cycle performance of rechargeable Zn/MnO2 cells. The batteries with aqueous ZnSO4 electrolyte can be cycled in the potential range of 1.0–1.9 V, where a two-step, two-electron charge-discharge reaction occurs. In order to improve the reversibility of Zn/MnO2 cells, MnSO4 was added into the ZnSO4 solution. The cycle performance has also been improved from a few cycles to one hundred cycles. However, the addition of MnSO4 with a high concentration can lower the pH value of the electrolyte, which leads to a serious hydrogen evolution occurring in Zn anode.
Different from the traditional primary Zn/MnO2 battery chemistry, the recent literature data showed that discharge and charge reactions of cathodic MnO2 can be reversible in an aqueous ZnSO4 or Zn(NO3)2 electrolyte and a so-called zinc ion battery (ZIB) chemistry has been proposed.14,15 In such a battery, the charge storage mechanism is based on the migration of Zn2+ ions between α-MnO2 cathode and Zn anode within a mild Zn(NO3)2 or ZnSO4 aqueous electrolyte. Because this novel rechargeable battery is based on the Zn anode, the formation of inactive dendrites or/and anodic products on the surface of Zn particles is also an inevitable problem, which results in the rapid capacity fading of zinc ion batteries.
In this paper, we develop a new approach to enhance the cycle performance of Zn anode in neutral aqueous electrolytes by adding activated carbon with rich and well-developed pore structure. The rich and well-developed pores of activated carbon are the ideal space for the deposition of Zn dendrites and other passivation products which are normally produced during the charge/discharge processes of Zn anodes. As a result, the surface of Zn particles remains neat and active, leading to a significant enhancement on cycle performance. In addition, the effect of activated carbon modification on the electrochemical performance of Zn anodes for rechargeable batteries has been investigated.
Experimental
Materials and batteries preparation
Amorphous α-MnO2 powders were prepared via a self-reacting micro-emulsion method as described in previous reports.14,16 To prepare the cathodes, α-MnO2 powders, acetylene blacks (Mitsubishi Gas Chemical Co.) and polyvinylidene fluoride (PVDF, Aladdin) binder were mixed in an appropriate ratio (7:2:1 in weight ratio) with adding some N-methyl-2-pyrrolidone (NMP, Aladdin). The mixtures were then stirred for 3 hours and coated on a piece of stainless-steel film (30 μm in thickness). Finally, they were dried at 85°C in vacuum, pressed at 10 MPa, and cut into small round electrodes (0.7 cm in diameter).
The preparation of Zn powder anodes is similar to MnO2 cathodes. For the unmodified Zn anode, Zn powder (99.5%, Aladdin), acetylene blacks (AB, Mitsubishi Gas Chemical Co.) and PVDF binder were mixed at 7:2:1 in weight ratio. For activated carbon (AC) -modified Zn anodes, the weight ratios of AC are 5%, 8% and 12%, respectively. Correspondingly, the weight ratios of Zn powder are 65%, 62% and 58%, respectively. AC used in this work was prepared from mesophase pitch through a simple pre-carbonization and chemical activation process.17 In order to evaluate the performance of Zn anode, the amount of MnO2 cathode material is much larger than that of Zn anode material. The effective mass loading of as-prepared Zn anode and MnO2 cathode material are about 1.8 mg cm−2 and 5.4 mg cm−2, respectively. In this work, the Zn anode without AC modification is denoted as "ZnAB", while the AC modified Zn anode is denoted as "ZnAB+AC". 1 mol L−1 aqueous ZnSO4 solution was used as the electrolyte. A microporous polypropylene membrane was used as separator. All the materials were assembled to button cells by a sealing machine.
Materials characterization
Structural properties of ZnAB and ZnAB+AC were characterized with a Rigaku D/max 2500/PC diffractometer (Rigaku Corp., Japan) (CuKα, λ radiation = 1.54056 Å) before and after charge/discharge test. The diffraction patterns were recorded over the 2θ angle of 10∼90° at 40 kV, 30 mA. The morphologies of the samples were determined by a field emission scanning electron microscopy (FE-SEM, HITACH S4800) at 10 kV. Elemental analysis was conducted with an energy dispersive spectrometer (EDS, Horiba 7593-H) which is attached to the SEM machine. N2 adsorption and desorption studies were carried out by an ASA P2010 (Micromeritics Company) surface area analyzer and the pore size distribution was determined by the density function theory (DFT).
Electrochemical characterization
Electrochemical performance of the as-prepared ZnAB and ZnAB+AC were examined based on galvanostatic testing of CR2032-type coin cells in the voltage range of 1.0 −1.9 V using a Land 2001A battery testing system at 25°C. All the Zn anodes were tested at a current density of 200 mA g−1 (about 1.2 C) with 100% depth of discharge.
Cyclic voltammogram (CV) measurements were carried out with a VMP3 multichannel electrochemical station (Bio-Logic-Science Instruments SA, France) at room temperature at a scanning rate of 0.1 mV s−1, shifting from −1.5 V to −0.5 V vs. SCE. A three-electrode system was employed with a MnO2 electrode as the counter electrode, the testing Zn electrode as the working electrode and a saturated calomel electrode (SCE) as the reference electrode. The electrolyte is 1 mol L−1 aqueous ZnSO4 solution.
Results and Discussion
Structural and compositional characterization
Activated carbon, a well-known carbonaceous material with good electrical conductivity and excellent chemical stability, has complex pore structure, large specific surface area, high adsorption capacity and high degree of surface reactivity. It has been widely used in many areas, such as water treatment, gas separation and energy storage.18–20 In this work, activated carbon was added into the unmodified Zn powder anode to improve the cycle performance of neutral rechargeable zinc ion batteries.
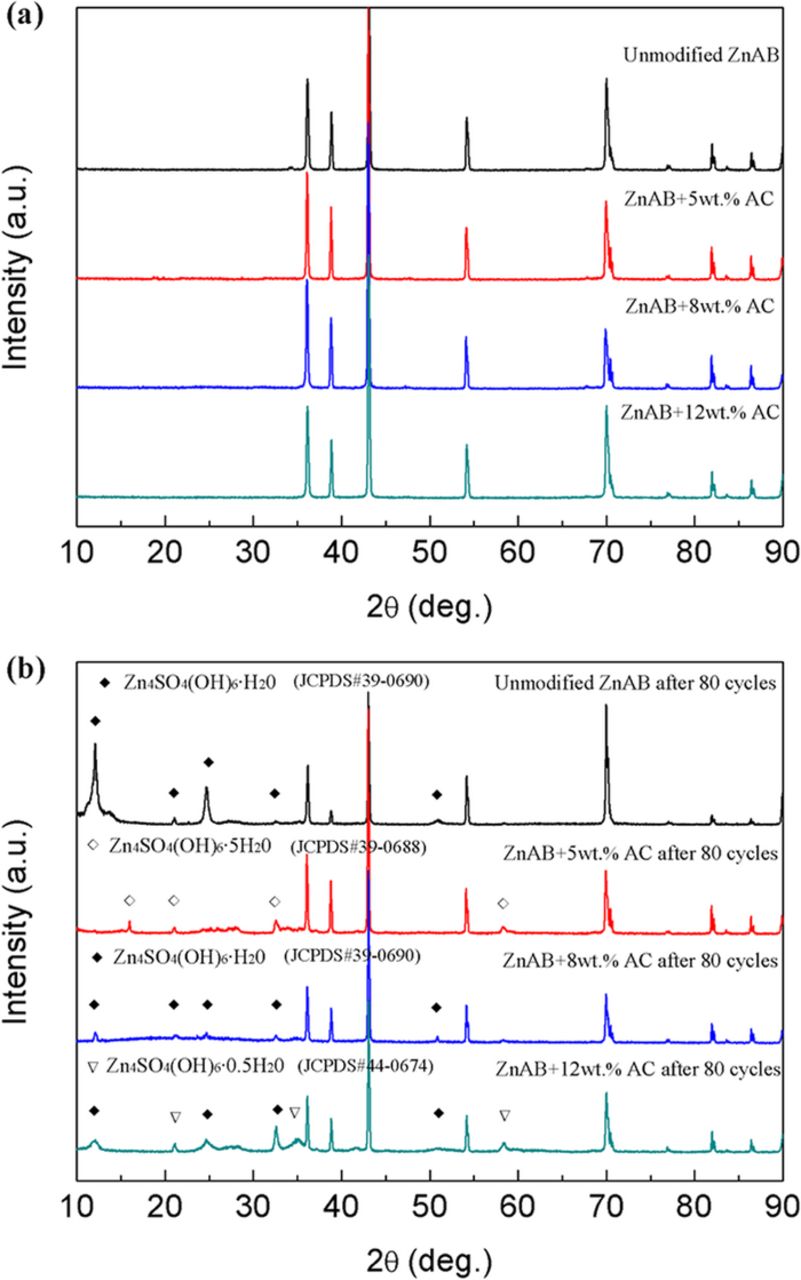
XRD patterns of the as-prepared ZnAB and ZnAB+AC were studied to examine the compositional difference (Fig. 1). Before the charge/discharge test, The unmodified ZnAB, ZnAB+5 wt.%AC, ZnAB+8 wt.%AC and ZnAB+12 wt.%AC exhibit similar XRD patterns and the diffraction peaks could be exclusively ascribed to Zn (JCPDS card No. 65–3358) (Fig. 1a). Due to the high peak intensity of Zn metal, no peaks of AC can be detected. In comparison with Zn anodes before the cycle test, it is clearly found that a new phase, which is characterized with peaks at 12.2, 24.6 and 50.8 degree, is detected in the unmodified ZnAB after 80 cycles (Fig. 1b). These peaks can be attributed to the basic zinc sulfate (Zn4SO4(OH)6·H2O, JCPDS card No.39-0690), which is an inactive and insoluble sulfate deposition.9 The accumulation of inactive Zn4SO4(OH)6·H2O on the surface of Zn particles could dramatically inhibit the reversible deposition/dissolution of zinc and limit the active material utilization, which is predicted to be responsible for the performance degradation of the neutral zinc ion battery. After 80 cycles, more kinds of basic zinc sulfates, Zn4SO4(OH)6·0.5H2O (JCPDS card No.44-0674) and Zn4SO4(OH)6·5H2O (JCPDS card No. 39–0688), have been detected in the AC-modified zinc anodes. It is noteworthy that the peak intensities of basic zinc sulfates in AC-modified Zn anodes after 80 cycles (Zn4SO4(OH)6·5H2O for ZnAB+5wt%AC; Zn4SO4(OH)6·H2O for ZnAB+8wt%AC; Zn4SO4(OH)6·H2O and Zn4SO4(OH)6·0.5H2O for ZnAB+12wt%AC) are greatly reduced, which means that the addition of AC could effectively reduce the deposition of basic zinc sulfates (Fig. 1b). As a result, the AC modification is expected to enhance the electrochemical performance of zinc ion batteries.
Figure 1. X-ray diffraction patterns of unmodified ZnAB and 5, 8, 12 wt% AC-modified Zn anodes before (a) and after 80 cycles (b).
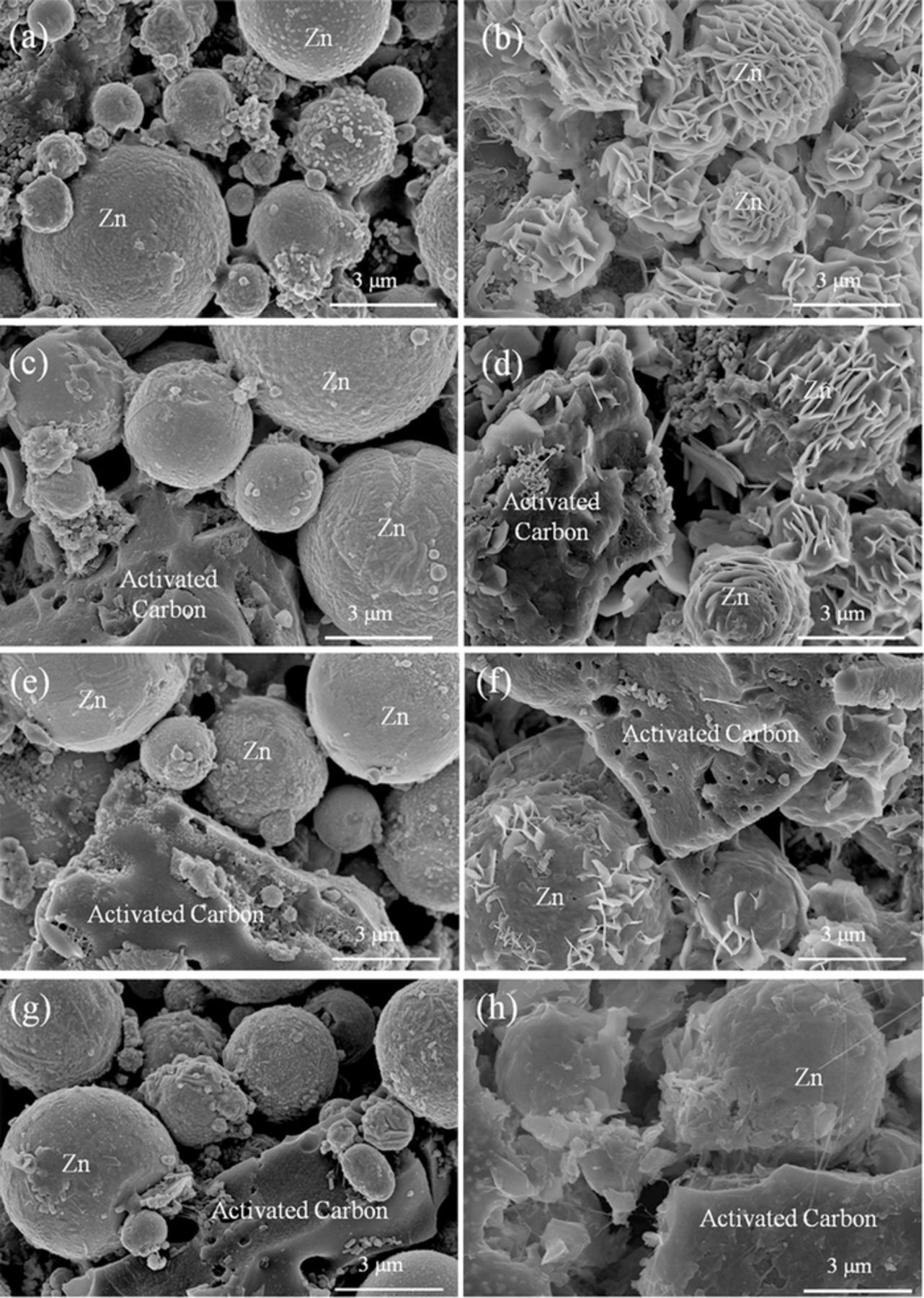
The morphologies of the unmodified Zn anode (i.e. ZnAB) and Zn anodes containing 5, 8 and 12 wt% activated carbon (i.e. ZnAB+AC) before and after cycles are presented in Fig. 2. The initial morphology of the Zn particles in ZnAB has an inerratic spherical shape with a smooth surface (Fig. 2a). However, after 80 charge/discharge cycles, the Zn particles are covered all over by Zn dendrites and the smooth spherical shape of Zn particles turns into a flower-like morphology (Fig. 2b). The formation of dendrites is also one of the serious problems in batteries based on Zn anodes because inactive dendrites block the transfer of ions and slow the ion diffusion process, leading to a pronounced anodic capacity fading. In addition, compared with a smooth spherical shape, the dendrites with sharp thin sheets covered on zinc particles increase the possibility of penetrating into the separator, which may result in an interior short circuit.8 Based on the XRD and SEM analysis, the capacity loss of this neutral zinc ion battery is attributed to the formation of inactive basic zinc sulfates (Zn4SO4(OH)6·nH2O) and Zn dendrites.
Figure 2. SEM images of (a) initial ZnAB, (b) ZnAB after 80 cycles, (c) ZnAB+5 wt% AC, (d) ZnAB+5wt%AC after 80 cycles; (e) ZnAB+8wt% AC, (f) ZnAB+8wt%AC after 80 cycles; (g) ZnAB+12wt%AC, and (h) ZnAB+12wt%AC after 80 cycles. The scale bar is 3 μm.
After the addition of 5wt% AC, it can be seen that although the Zn particles after cycles are still covered by sheet dendrites, the coverage ratio is reduced and a small part of the Zn particle surface is still smooth (Fig. 2d). As the weight ratio of activated carbon increases to 8wt% and 12wt%, Zn particles become much more neat and little Zn dendrites can be found around their surface after the charge/discharge test (Figs. 2f, 2h), which indicates that most of the Zn particles still remain active. It is worthy to notice that the surface of activated carbon is also covered by a layer of deposition, which means that the activated carbon is a suitable place to accommodate the deposition of zinc sulfate and Zn dendrites.
To confirm the activated carbon particles are covered by Zn deposition after cycle test, the EDS elemental mapping of ZnAB+12wt%AC before and after cycles were determined. As shown in Fig. 3a, the Zn element is only distributed around Zn particles before the charge/discharge test. However, after 80 cycles, the Zn element nearly covers the entire electrode region (Fig. 3b). In particular, a higher concentration of Zn element is found in the area of activated carbon, proving that the activated carbon particles are the ideal sites for the accommodation of anodic Zn deposition.
Figure 3. EDS images and corresponding Zn and C elemental maps of ZnAB+12wt%AC anode before (a) and after (b) charge/discharge cycles.
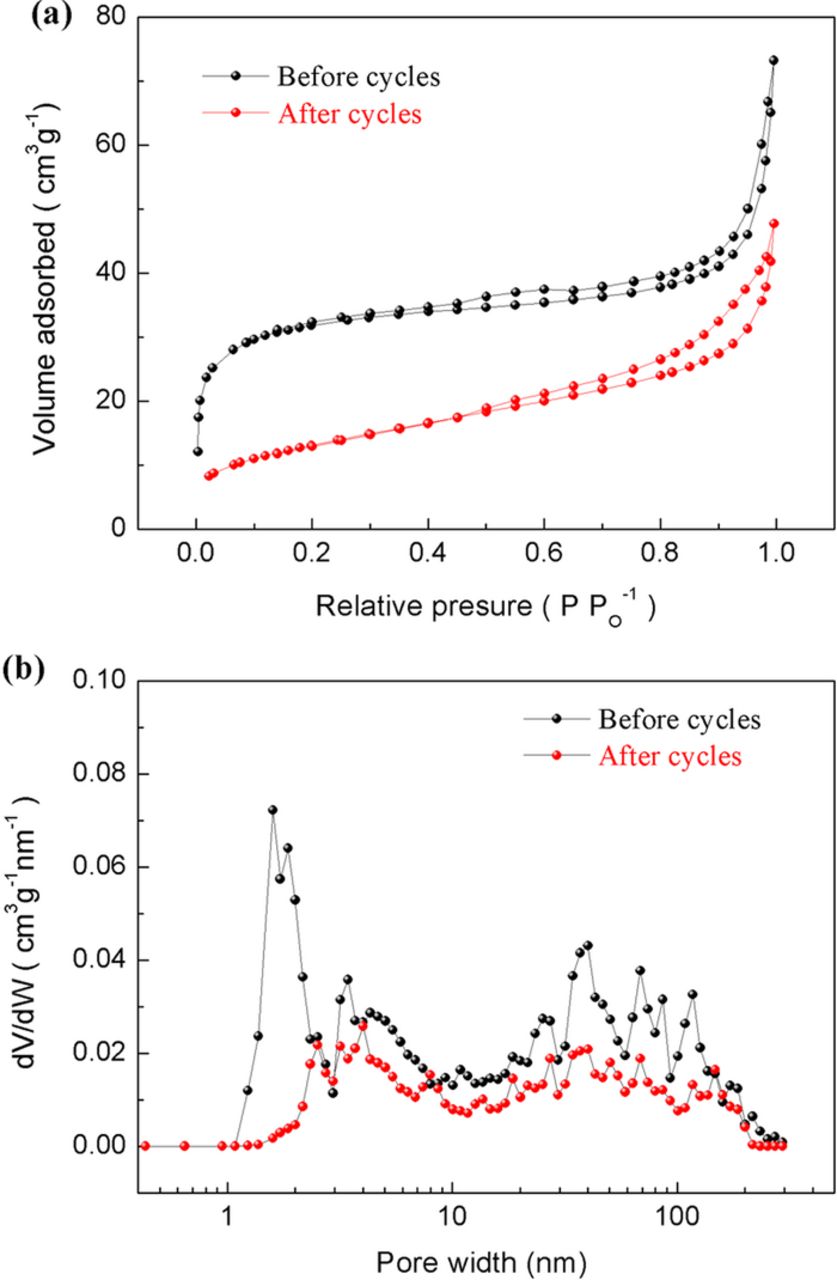
In order to further determine whether or not the pores of activated carbon could hold the deposition, N2 adsorption and desorption studies was carried out for ZnAB+12wt%AC before and after cycling. The nitrogen adsorption/desorption isotherm curves and the pore size (W) distribution are shown in Fig. 4.
Figure 4. The nitrogen adsorption/desorption isotherms (a) and pore size distribution (b) of ZnAB+12wt%AC before and after cycles.
According to IUPAC nomenclature, micropores are less than 2 nm in diameter, mesopores are between 2 to 50 nm in diameter and macropores are greater than 50 nm in diameter. As shown in Fig. 4a, the adsorbed volume rises sharply at low relative pressures (P/P0<0.1) before the charge/discharge test, revealing that there are micropores in the AC-modified Zn anodes. The presence of these micropores is attributed to the addition of activated carbon. However, after 80 cycles, there is no significant increase of the adsorbed volume at low relative pressures, which indicates the micropores in the AC-modified Zn anodes are filled with anodic products after the charge/discharge test.
Figure 4b shows the variation of pore size distribution evaluated by the density function theory (DFT). Before cycles, there is a very wide pore size distribution ranging from micropore to macropore for ZnAB+12wt%AC. In the micropore region, there is a sharp peak emerging at 1.5 nm, indicating the microporous nature of activated carbon. However, after 80 cycles, the pore size only locates in the mesopore and macropore region and the pore volumes of mesopores and macropores experience a decrease. This result clearly proves the disappearance of micropores. On the other hand, the BET surface areas of ZnAB+12 wt.%AC before and after the cycle test are 110.1 m2 g−1 and 47.8 m2 g−1, respectively. The disappearance of micropores together with the decrease in surface area clearly indicate the deposition of Zn dendrites or other products into the pores of activated carbon instead of on the surface of Zn particles. Due to the absence of inactive Zn dendrites and passivation on the surface of Zn particles, the Zn anode is still highly active, which leads to the enhancement on the capacity retention during cycling. This result is in accordance with SEM analysis and the elemental distribution maps.
Electrochemical performance
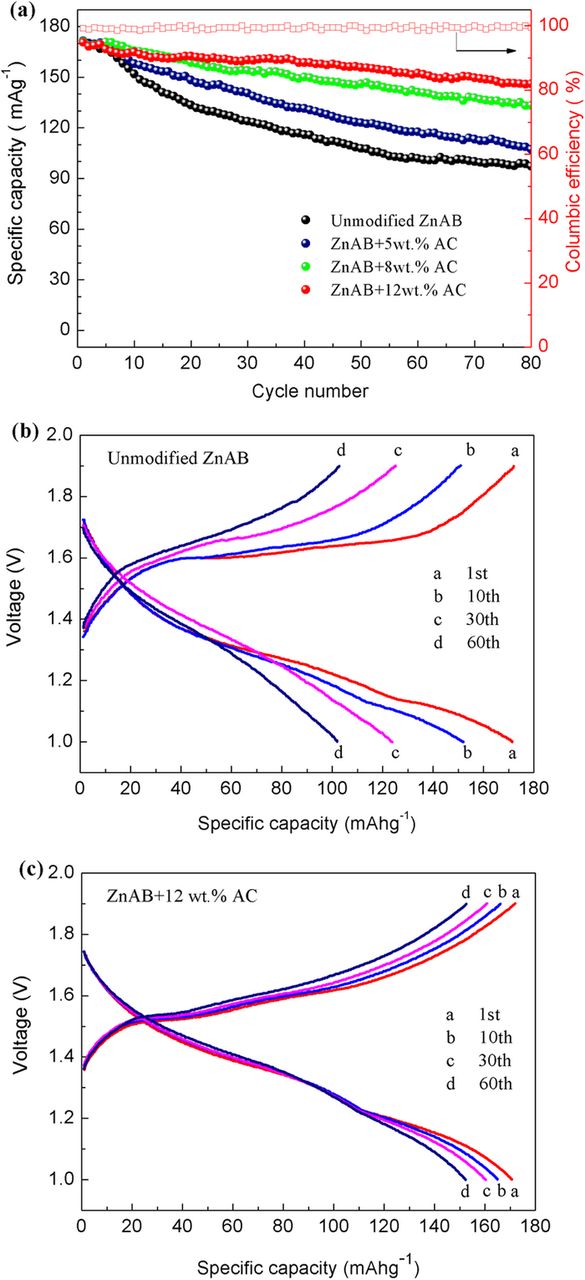
The cycle performance of the unmodified ZnAB and Zn anodes containing 5, 8 and 12 wt% AC is presented in Fig. 5. For ZnAB, the initial discharge capacity is 171.5 mAhg−1, which declines significantly with increasing cycle numbers. After 80 cycles, the discharge capacity reduces to 97.2 mAhg−1 with the capacity retention of 56.7%. This is a typical phenomenon occurring in rechargeable Zn/ MnO2 batteries based on Zn anodes, which is due to the formation of zinc dendrites and insoluble anodic products on the electrode surface.7
Figure 5. (a) Cycling performance of rechargeable zinc ion batteries with the unmodified ZnAB and ZnAB+AC (charge/discharge at 200mAhg−1). The charge/discharge curves of unmodified ZnAB (b) and ZnAB+12wt%AC (c) tested at the 1st, 10th, 30th, 60th cycle.
However, after adding activated carbon into Zn anodes, the cyclic stability of the cells improves remarkably in comparison with the pristine Zn anode. It is found that as the weight percent of activated carbon increases from 5 wt%, to 8wt%, and further to 12wt%, the cycle performance of corresponding zinc ion batteries becomes better. The discharge capacity of ZnAB+12wt%AC is the most stable and its columbic efficiency is nearly 100% throughout the charge/discharge processes, as shown in Fig. 5a. The capacity retentions of ZnAB+5wt%AC, ZnAB+8wt%AC, and ZnAB+12wt%AC after 80 cycles are 62.5% (107.2 mAhg−1), 77.9% (133.3 mAhg−1) and 85.6% (146.1 mAhg−1), respectively.
As mentioned above, the pores of activated carbon can accommodate the deposition of Zn dendrites and insoluble anodic products. If the adding amount of activated carbon is less, there are not enough pores to contain the deposition. Therefore, a large part of Zn particles will be covered by inactive Zn dendrites and basic zinc sulfates. However, if the adding amount of activated carbon is large enough to accommodate the deposition, the surface of Zn particles will remain neat and active even after tens of cycles. It explains the enhancement effect of activated carbon on the cycle performance of Zn anodes. But it should be noted that the excessive activated carbon can result in the remarkable decrease in the energy density of zinc ion battery. As shown in the Supporting Information (Fig. S1), when the AC content is further increased to 15wt%, the electrochemical performance of the AC-modified Zn anode begins to decline. The specific discharge capacity of ZnAB+15wt%AC declines to 138.1 mAhg−1 and its capacity retention after 80 cycles is 82.3%, slightly lower that of ZnAB+12wt%AC. So the activated carbon should be added to an appropriate extent.
The typical charge/discharge curves of ZnAB and ZnAB+12wt%AC tested at the 1th, 10th, 30th, 60th cycle are displayed in Figs. 5b–5c. The ZnAB+12wt%AC anode shows relatively higher discharge plateau voltage (1.23 V for the 1st discharge cycle) and lower charge plateau voltage (1.52 V for the 1st charge cycle) while the corresponding discharge and charge potentials of ZnAB are 1.16 V and 1.59 V, respectively. The charge/discharge potential difference for ZnAB+12 wt.%AC is 290 mV, which is much lower than the value of 430 mV for ZnAB, suggesting that the polarization of ZnAB+12 wt.%AC is much less. In addition, it is also found that the charge/discharge curves of ZnAB+12 wt.%AC are much more stable than those of ZnAB as the cycle number increases.
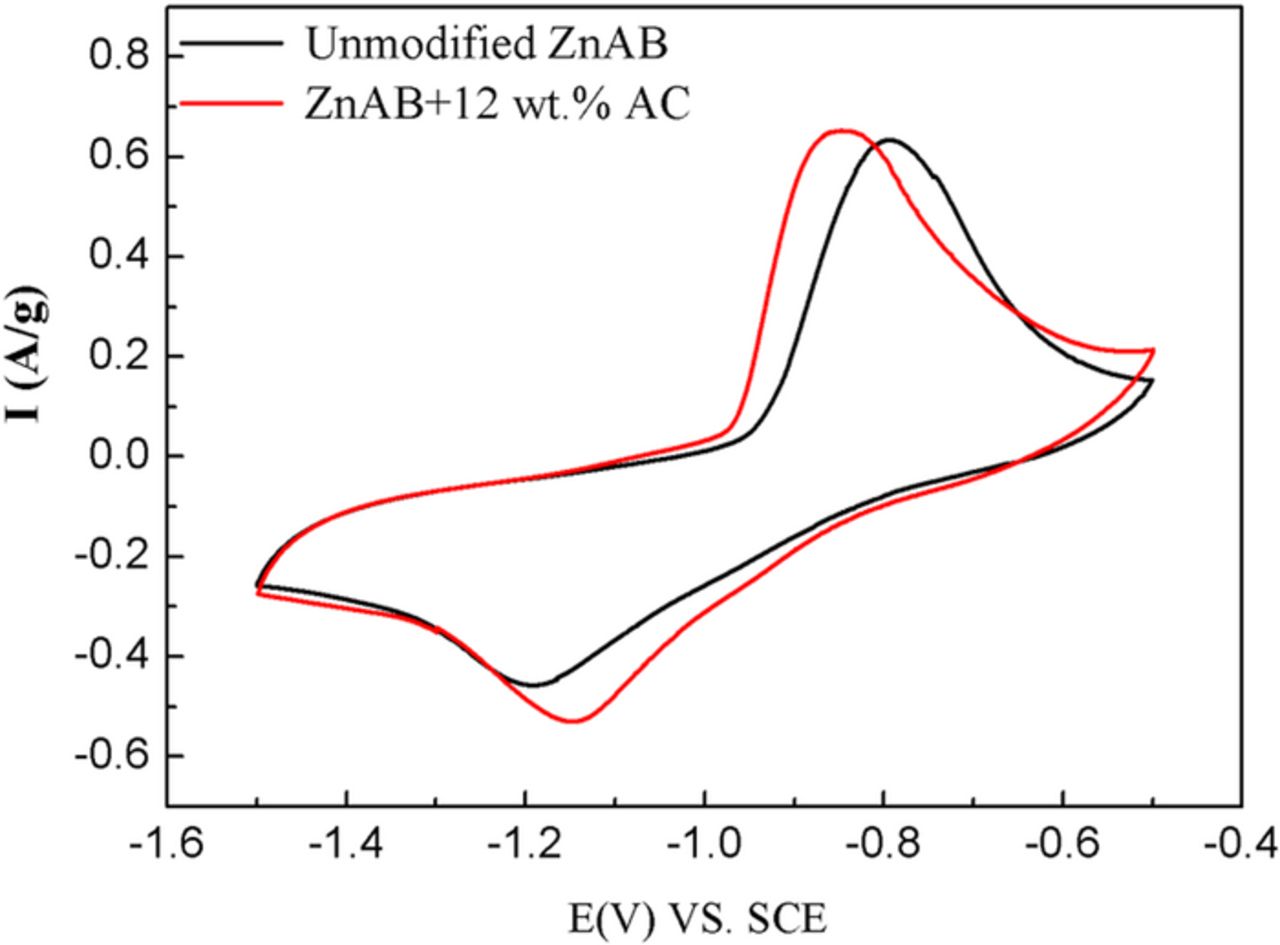
In order to further understand the influence of activated carbon modification on electrochemical reaction kinetics of Zn anode, cyclic voltammetry (CV) tests were performed at a scanning rate of 0.1 mV s−1 within a potential range from −1.5 V to −0.5 V vs.SCE. CV curves of the unmodified ZnAB and ZnAB+12wt%AC are presented in Fig. 6. A pair of redox peaks is observed from each CV curve, which indicates that only one well-defined reduction–oxidation reaction occurs in the electrochemical process. This redox couple should be associated with the deposition and dissolution of Zn2+ ions into/from the Zn anode. The voltage difference between the anodic and cathodic peaks (ΔEp) reflects the degree of polarization of the electrode. The ZnAB+12 wt.%AC anode has a much lower ΔEp value (0.30 V) and a relatively larger peak current density (-0.53Ag−1) compared with the unmodified ZnAB (0.39 V and -0.46 Ag−1). Therefore, the ZnAB+12 wt.%AC anode is less polarized and has faster electrochemical reaction kinetics.
Figure 6. CV curves of the unmodified ZnAB and ZnAB+12wt%AC at a scanning rate of 0.1 mV s−1.
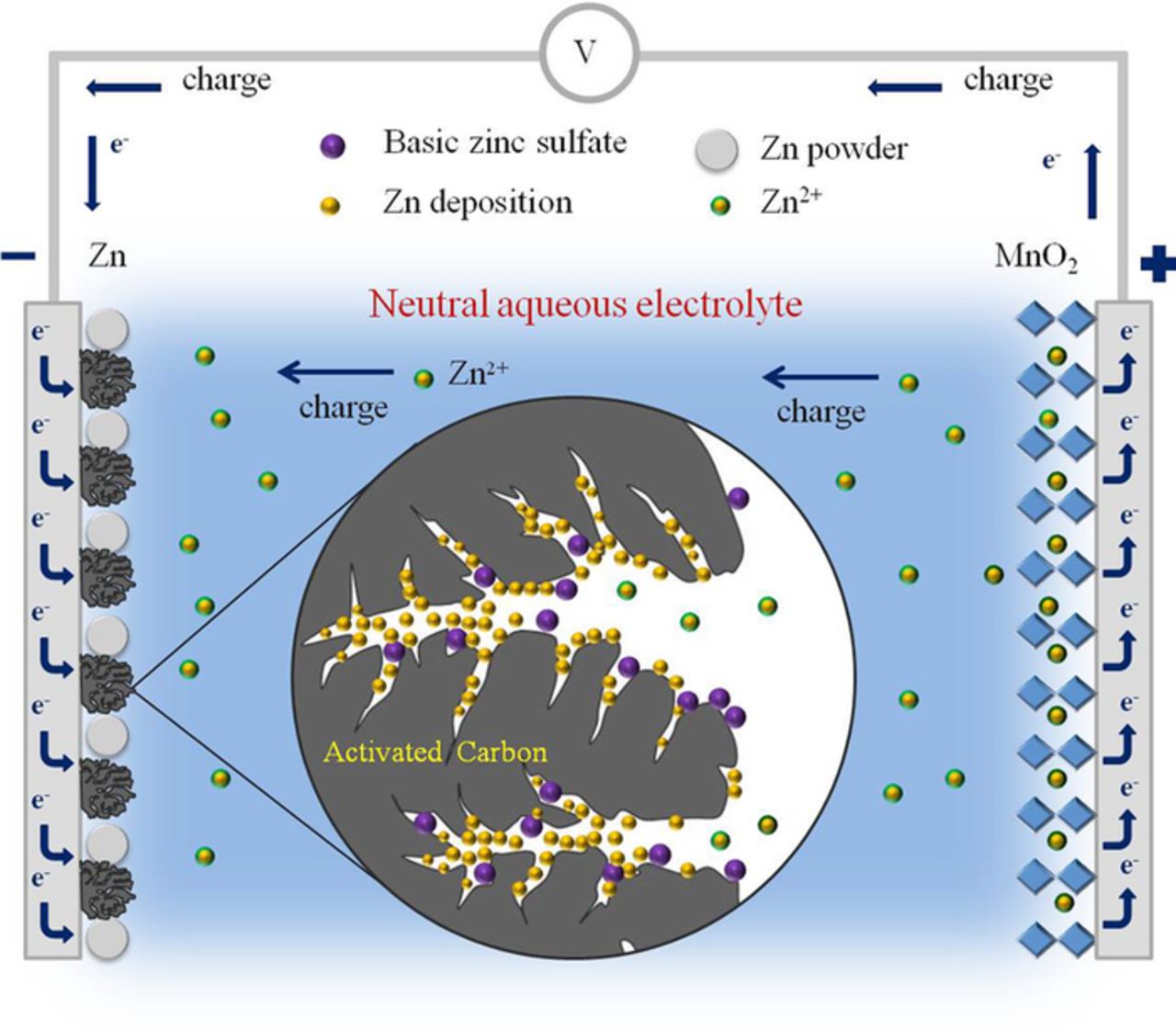
From the above discussion, we therefore come up with an enhancement mechanism of AC on the cycle performance of neutral rechargeable zinc ion batteries. As the schematic illustration shows in Fig. 7, the AC particles in the anode are filled with electrons during the charge process. Several studies have provided evidence for the adsorption of Zn2+ ions on AC in aqueous solutions.21–23 So when activated carbon is added into the Zn anode, Zn ions can be absorbed and migrate into the pores of AC during charging. Then they can combine with electrons and other negative ions to deposit. So the deposition of inactive anodic products are preferential to occur in the rich and well-developed pores of activated carbon rather than on the surface of zinc particles, thus keeping the Zn particles neat and active even after tens of cycles. As a result, the cycling reversibility of the zinc anode is greatly enhanced by activated carbon modification.
Figure 7. The schematic illustration for the deposition of anodic products in activated carbon during the charge process.
Conclusions
The cycle performance of the neutral zinc ion battery based on a Zn anode has been improved significantly by adding activated carbon into the Zn anode. The capacity retentions of the batteries without and with 12wt% activated carbon in Zn anodes are 56.7% and 85.6% after 80 cycles, respectively. The addition of activated carbon can suppress the formation of inactive basic zinc sulfate (Zn4SO4(OH)6·nH2O) characterized by the XRD diffraction. Results show that due to the presence of activated carbon, the deposition of anodic products is preferential to occur in the pores of activated carbon rather than on the surface of Zn particles. As a result, the electrochemical reaction kinetics and reversibility of the zinc anode have been greatly improved by activated carbon modification, leading to the enhancement on the cycle performance of batteries based on Zn anodes.
Acknowledgments
This work was financially supported by National Key Basic Research Program of China (No. 2014CB932400), China Postdoctoral Science Foundation (No. 2013M530617), National Nature Science Foundation of China (51202121 and 51232005), NSAF (grant No. U1330123), Shenzhen Technical Plan Project (Nos. JCYJ20120619152808478 and JCYJ20130402145002382, JCYJ20130402145002425) and Guangdong Province Innovation R&D Team Plan for Energy and Environmental Materials (No. 2009010025).