Abstract
Functional properties of flexible reflective electrochromic composites comprised of polyaniline-coated metallized textile were investigated in this study. Polyaniline was deposited electrochemically onto metal-plated textile fabric and the resulting composites were investigated by electrochemical means as well as by optical-digital colour analysis. Surface morphology and microstructure were evaluated using scanning electron microscopy. Electrochromic performance of the conducting textile and polyaniline composites was optimized by tuning the applied electrochemical switching parameters. Electrochromic textile composites exhibited reversible colour change with good visual contrast between the coloured and bleached states. Functional stability of electrochromic metal-plated textile/polyaniline composite was evaluated by continuous switching between the colored states for 100 cycles. Herein presented concepts might find future use in the development of flexible, colour changing visual interfaces and/or wearable technology, the Internet of things (IoT) devices and optical sensors.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Electronic textiles and smart wearable devices are undergoing a rapid development and actively entering user market during the recent years. Sensors and sensing systems detecting pressure,1 temperature,2 strain,3 triboelectricity,4 and touch gestures,5 as well as disease biomarkers and cellular metabolites, including glucose, lactate, and ascorbic acid6 have been successfully integrated into textile fabrics. Alongside the available variety of sensing technologies, strategies for providing the output of sensory data or otherwise interact with the wearer should be developed and implemented.
The most common case for interacting with the user of wearable technology, including commercial projects that recently appeared on the market, such as Google ATAP project "Jacquard", relies on an external smart device, i.e. a smartphone for providing an interface with wearable garment-embedded technology. To enable electronic textiles function as fully independent technology, fabric-integrated visual interfaces must be developed and brought to use. However, there are many challenges to be addressed. Wearable visual interface component should be integrated seamlessly, maintain comfort and wearability while sustaining mechanical and chemical stress throughout wearing and washing. The multitude of issues to be addressed presents a big challenge for technology developers.
Different methods of presenting a controllable visual output to the wearer using textile-embedded displays exist. Most popular methods include light emitting,7–9 thermochromic,10,11 electrophoretic, or electrochromic12 displays. The first two approaches suffer from the lack of bistability—i.e. the ability to maintain the displayed coloured state without external power source, therefore offering lower electric power effectiveness. Very few cases of electrophoretic display devices for electronic textile applications have been reported to this date, stemming from the fact that implementation of such display technology on substrates other than those of flat sheet geometry remains challenging. On the other hand, electrochromic displays offer a low power consumption combined with sufficiently good bistability and a large variety of different feasible substrates, including paper, leather, textiles.13 In fact, initial prototypes of electrochromic display wielding garments have already been developed and presented.14,15
Due to a great communicative potential of colour changing displays, the possible field applications of electrochromic technology for smart textiles is indeed vast, ranging from camouflage16 to fabric-integrated visual interfaces for displaying information or sending visual signals.17
Several early studies report chemical synthesis and observation as well as characterisation of electrochromic properties of textile fabrics and conducting polymer composites.18,19 However, such composites often suffer from limited long-term stability and long switching times, owing to the low electric conductivity of the resulting electrochromic fabrics.20 Some efforts were made to address this issue by sputtering conducting indium-tin oxide21 or by printing the silver electrodes directly onto the fabric.12
The applications of polyaniline (PANI) have been widely reported for various (spectro)electrochemical22 and optical sensor23 platforms. Upon applying electrochemical potential, PANI is able to undergo reversible oxidation and reduction reactions, which give rise to new light absorption bands, resulting in the colour change of the polymer layer. Due to these characteristics, PANI stands out as an excellent material for demonstrating proof-of-concept principles and devices owing to its low price and great versatility, as allowed by chemical modification of the aniline monomer24 and/or the modifications of PANI through co-polymerization with different monomers.25 The herein presented work is focused on the development of electrochromic textile composite, consisting of conducting metal-plated polyester textile (metal-textile) and electrochemically deposited conducting polymer polyaniline. By using metal-plated polyester fabric, high electric conductivity is achieved, allowing significantly shorter electrochromic switching times of the resulting electrochromic composites.
Materials and methods
Materials
Polyester plain woven fabric plated with nickel-copper-nickel (technical code "PES/Ni-Cu-Ni", further in this paper referred as "metal-textile"), which is typically used for electromagnetic shielding applications, with high electric conductivity (0.03–0.05 Ω sq−1) and 0.08–0.09 mm thickness was used as a substrate for electrochromic conducting polymer deposition. Chemical reagents aniline (CAS number: 62-53-3, "reagent grade" from Fluka) was used after double distillation, sulfuric acid (98%, CAS number: 7664-939, purchased from Carl Roth) was used as received. Deionized water was used in preparation of all aqueous solutions.
Apparatus
Electrochemical measurements were carried out using potentiostat/galvanostat Autolab PGSTAT128N from Eco-Chemie (Utrecht, The Netherlands). Video capturing for digital image characterization was performed using a mobile smartphone OnePlus 6 camera Sony Exmor IMX519 (16 MP, f/1.7 4.25 mm, OIS + EIS, ISO 250). Surface morphology and microstructure analysis was performed with scanning electron microscope TM3000 from Hitachi (Tokyo, Japan).
Methods
Electrochemical polymerization was carried out using three electrode system, where silver wire was used as a pseudo-reference electrode, platinum wire served as a counter electrode and metal-plated polyester textile—as a working electrode. Electrochemical deposition of conducting polymer polyaniline was performed by 8 potential cycles in the range from 0.1 V to +1.0 V, at 100 mV s−1 potential sweep rate, in water-based polymerization bulk solution containing 0.2 M aniline and 0.5 M sulfuric acid. The obtained textilepolyaniline composites were then thoroughly washed with deionized water in order to remove remnant monomer species as well as loosely bound PANI-fragments.
Electrochromic colour change demonstrated by metal-textile/PANI composites was registered by video recording under stable experimental conditions and video captures were investigated using image analysis software. Digital image characterization of video captures was performed using ImageJ (version 1.52a) open source image processing software package, developed at National Institutes of Health (USA), and the Laboratory for Optical and Computational Instrumentation (LOCI, University of Wisconsin, USA). Every analysed pixel is registered as the intensities of three primal colours: red (R), green (G) and blue (B). Intensity can be evaluated as number from 0 to 255, where RGB = (0; 0; 0) would mean absolute black and RGB = (255; 255; 255) absolute white colour. During analysis with ImageJ software package, R, G, B channels of each pixel are separated and the change of their intensities in time registered. For easier monitoring of general colour change, mean gray value (MGV), defined as arithmetical mean of three primary colour channels: MGV = (R+G+B)/3, was measured. The captured videos were split into stacks of images, in which the region of interest was selected, cut and analysed by measuring the average intensity of RGB colour channels. Due to the known frame rate of the video captures, it was possible to perform time-resolved colour analysis of electrochromic metal-textile/PANI composites in order to calculate characteristics of electrochromic kinetics, such as colouration and/or bleaching time. To ensure constant lightning conditions, all experiments were carried in special recording setup with constant LED lighting and background, providing stable experimental video capturing conditions.
Colour values in CIE L*a*b* colour space were calculated from RGB values using CIE D65 illuminant and 2° observer. Optical contrast (ΔE) was calculated from CIE L*a*b* colour values according to the following equation:
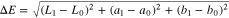
where subscript indexes indicate CIE L*a*b* colour values of electrochromic layer in initial or end coloured state.
Results and Discussion
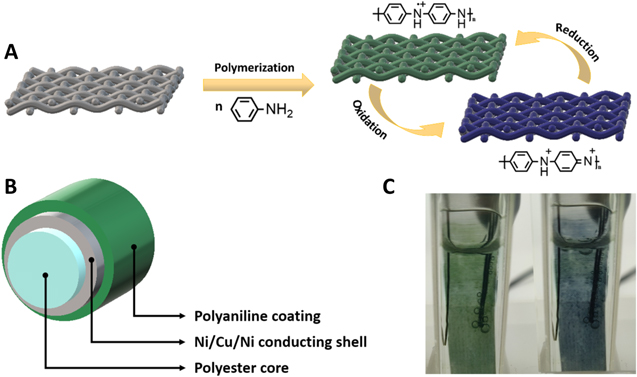
Cyclic voltammogram of PANI electrochemical deposition and cyclic voltammogram of deposited PANI in monomer-free solution are presented in Figs. 1S and 2S in Supporting Information (available online at stacks.iop.org/JES/167/155515/mmedia). During electrochemical synthesis, polyaniline is deposited onto the surface of metal-plated textile, thus forming the visible outer layer, capable of reversibly changing colour from green to blue, upon applied electrochemical potential (Fig. 1a). The schematic cross-section of the obtained electrochromic PANI-metal textile composites is depicted in Fig. 1b, showing a polyester core plated with a thin metal layer, subsequently coated with an electrochromic conducting polymer. Photographic images of the obtained electrochromic textile composite in their respective oxidized (green) and reduced (blue) states are shown in Fig. 1c.
Figure 1. Schematics of electrochemical deposition of PANI onto conducting textile electrode and the principle of reversible electrochromic switching (a), cross-section schematics of electrochromic textile composite, consisting polyester fiber core, Ni/Cu/Ni conducting metal plating and outer polyaniline coating (b), photographic image of polyaniline and PES-Ni/Cu/Ni composite observed electrochromic textile colour change—left (green) in the electrochemically reduced state, right (blue) in the oxidized state (c).
Download figure:
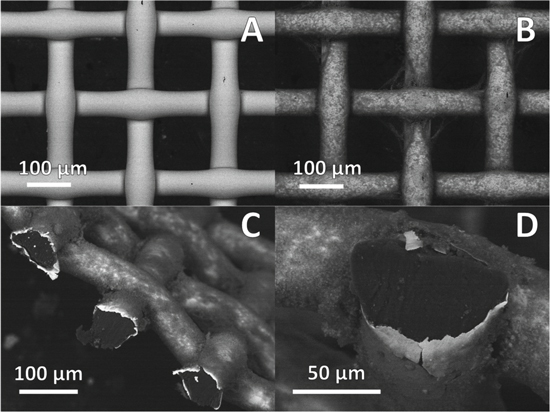
Standard image High-resolution imageTo explore the morphology and microstructure of electrochemically deposited polyaniline and PES-Ni/Cu/Ni textile composites, scanning electron microscopy (SEM) analysis was carried out. SEM micrograph of Ni/Cu/Ni-plated polyester reveals a uniform metal plating evenly surrounding the polyester surface with a few minor cracks or defects in the metal layer, as seen in Fig. 2a. SEM micrograph of metal-textile/PANI composite is presented in Fig. 2b. It reveals that polyaniline forms a fuzzy polymeric layer surrounding the whole surface of textile metal plating and therefore providing a wide viewing angle for electrochromic effect. Some loosely attached polyaniline strands formed between the interlacing threads can be observed, indicating potentially weak points where mechanical damage to the electrochromic layer might first occur when subjected to mechanical stress during the wear. Such observations suggest that different electrochromic material deposition methods or parameters, forming a more uniform active layer, capable of maintaining structural integrity under mechanical stress should be considered in the future studies. Cross-section SEM images of conducting textile and polyaniline composite are presented in Figs. 2c and 2d. A non-conducting polyester thread core is seen surrounded by a thin metal plating, providing metal-textile/PANI fabric composite with high electric conductivity, necessary for electrochromic switching applications. Although electrochemically deposited polyaniline layer is not uniform, it appears to coat most of the visible surface of conducting textile metal plating layer.
Figure 2. Scanning electron micrographs of Ni/Cu/Ni plated polyester (PES) woven textile (a), Ni/Cu/Ni plated PES textile electrochemically coated with polyaniline (b), side view of metaltextile/PANI composite based fabric (c), cross-section of metal-textile/PANI composite based fabric with electrochemically deposited polyaniline layer (d).
Download figure:
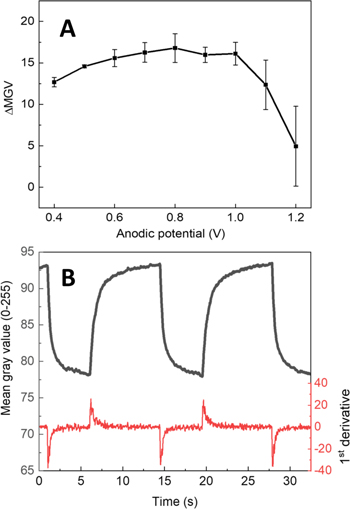
Standard image High-resolution imageTo characterize the electrochromic properties and to determine the optimum electrochromic switching conditions for polyaniline/conducting textile composites, various parameters of electrochromic switching were tested and optimized. To determine the optimum anodic electrochemical potential, required for electrochromic switching, we performed experiments with varying applied upper voltage (Fig. 3a).
Figure 3. Mean gray value intensity measurements of polyaniline and textile composite (a), The dependence of change in mean gray value on upper switching potential of polyaniline-conducting textile composite (b).
Download figure:
Standard image High-resolution imageIt was found that oxidative potential values as low as +0.4 V vs Ag/Ag+ were able to induce a significant change in colour, however, the change in colour upon applying electrochemical potential for conducting textile/PANI increased gradually, until it reached +0.8 V vs Ag/Ag+, where it exhibited the highest visually observable change. A further increase in the upper electrochromic switching potential value resulted in the rapid degradation of electrochromic performance, as evidenced by the decreasing change in colour, due to polyaniline undergoing an over-oxidation at higher electrochemical potential. Despite the fact that the highest optical contrast was achieved by switching the electrochemical potential between −0.1 V and +0.8 V vs Ag/Ag+, it has proven to be an important and beneficial trade-off to use lower anodic electrochemical potential values for electrochromic switching. In such way, sacrificing a negligible fraction of optical contrast, in turn gains a significant increase in long-term electrochromic switching stability. For this reason, lower than +0.8 V vs Ag/Ag+ potential values for electrochromic switching were used for all further experiments.
It has long been observed that the kinetics of cathodic process (colouration) of polyaniline is significantly slower than that of anodic process (bleaching).26 This trend is also observed in our optical measurements and reflected in Fig. 3b, where the first order derivative of the mean gray value with respect to time is calculated. It is easily noticeable that colouration process exhibits a much more rapid change in colour compared to bleaching process, as it is indicated by a higher amplitude peak in the first order derivative graph. In turn, this finding let us conclude that under similar period of time, when polarity of the applied electrochemical potential is reversed, PANI layer is not capable of returning to its initial colouration state, consequently leading to poorer electrochromic performance. Therefore, in order to reach a stable electrochromic switching equilibrium, the duration of applied oxidation and reduction potentials should be accordingly balanced.
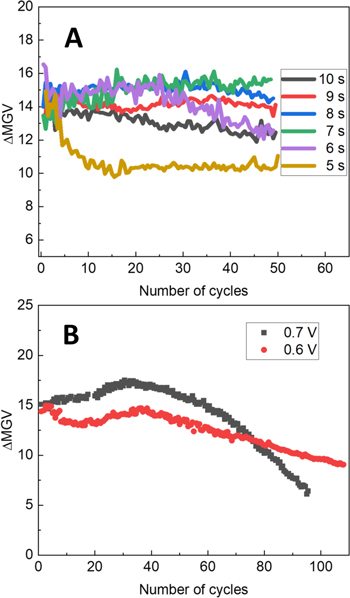
To achieve this, conducting textile coated with PANI was tested for 50 electrochromic switching cycles under different cathodic potential durations, ranging from 5 s to 10 s, while anodic potential was kept for constant duration of 5 s. Measuring 50 switching cycles allowed us to evaluate not only the initial performance of the electrochromic textile, but also to assess the tendencies of colour change intensity, caused by varying the cathodic potential duration. As it is observed in Fig. 4a, with an increase of cathodic potential duration from 5 s to 6 s, significant improvement of initial colour change of PANI-based electrochromic textile is observed. However, upon reaching 25 cycles of electrochromic switching, the electrochromic performance of herein reported metal-textile/PANI composite starts to deteriorate. The optimum colour change behaviour is achieved when the oxidation time was 5 s and the reduction time was set to 7 s, as no observable loss in electrochromic performance was detected for the first 50 cycles. Increasing the duration of applied cathodic potential even further leads to negative impact on the amplitude of change in colour, likely stemming from the effect of the opposite origin, since under these conditions larger fraction of PANI layer gets reduced than oxidized in a single electrochromic switching cycle. Based on these findings, the anodic potential for electrochromic switching of polyaniline was kept for 5 s, while the cathodic potential duration was set to 7 s in further experimental trials.
Figure 4. Electrochemically induced change in mean gray value dependence on the duration of applied cathodic potential (a), electrochromic performance stability of metal-textile/PANI composite under repetitive switching between −0.1 V cathodic and +0.6 V and +0.7 V anodic potentials vs Ag/Ag+ (b).
Download figure:
Standard image High-resolution imageFollowingly, we attempted to evaluate long term switching stability of the electrochromic metal-textile/PANI composite, which is a crucial property often limiting the potential application of electrochromic technology. To evaluate the operational switching stability of metaltextile/PANI composite, we carried out 100 switching cycles between −0.1 V and +0.7 V vs Ag/Ag+, while recording the visual changes in colour. The results of this trial are presented in Fig. 4b. It is seen, that polyaniline layer exhibits a fluctuating but stable electrochromic performance until 50 switching cycles, subsequently followed by a steady decrease in colour change amplitude after 50 switching cycles. Only 50 percent of optical contrast remained after 100 switching cycles when switching between −0.1 V and +0.7 V vs Ag/Ag+. On the other hand, we then attempted to lower the anodic switching potential value even further, performing functional stability measurements while repeatedly applying −0.1 V for 7 s and +0.6 V for 5 s. As expected, we found that polyanilinebased electrochromic textile composite exhibited lower optical contrast, nevertheless, it suffered a lower rate of optical modulation performance degradation under repetitive switching. This also provides an additional proof that degradation of electrochromic performance of polyaniline is due to anodic processes taking place at higher potentials. Additionally, the cost production of such electrochromic textile composites was evaluated. The laboratory scale production cost was estimated to stand at around 40 Euros per square meter, with an approximated cost being circa 5 times lower in case of scaled up production. In turn, this leads us to conclusion that in spite of affordable production cost, polyaniline only works great for proof-of-concept demonstration of electrochromic textile composites, finding limited use in practical applications, where a much better operational stability is required.
In Table I summarized colour values of electrochromic switching states and switching durations between those states of metal-textile/PANI composite are presented. It is seen that reaching 90 percent of optical contrast (colour difference) from reduced to oxidized (coloration) states of PANI takes 2.1 ± 0.3 s, which is significantly shorter period compared to the switching in the reverse direction (bleaching)—3.3 ± 0.3 s. Optical contrast (ΔE) was calculated from CIE L*a*b* colour values according to the Eq. 1. A higher optical contrast value indicates a greater distance between the points denoting specific colours in the colour space and, therefore, a better visually perceived difference between the two coloured states. In our study, a relatively high value of 27.6 for optical contrast is achieved, which leads to the conclusion, that metal-textile/PANI composite possesses distinctly coloured electrochromic switching states that are well perceived and distinguished by human vision.
Table I. Electrochromic switching parameters, colour and kinetic values (n = 10) for different switching states of metal-plated textile/PANI composite.
| Start Potential vs Ag/Ag+ | Endpotential vs Ag/Ag+ | Initial Color RGB | Initial Color CIE L*a*b* | Optical Contrast (ΔE) | Switching Time (90% ΔE) | |
|---|---|---|---|---|---|---|
| −0.1 V | +0.7 V |

|
109 112 73 | 46.26 –8.57 21.82 | 27.6 | 2.1 ± 0.3 s |
| +0.7 V | −0.1 V |

|
71 82 80 | 33.80 −4.37 −0.54 | 3.3 ± 0.3 s | |
Conclusions
In this study we report a preparation, characterization and optimization of electrochromic textile based on metal plated synthetic fabric and conducting polymer polyaniline (PANI) composite. Electrochromic conducting polymer PANI layer was formed electrochemically on conducting woven textile substrate and exhibited reversible colour change behaviour. Smart handheld visual experimental data acquisition in combination with digital image characterization was used for evaluating the electrochromic performance of the conducting textile and polyaniline composites, taking advantage of the abundance and technological advancements of smartphone video capturing technology. Various factors affecting colour change intensity and stability, such as electrochemical potential values and pulse durations have been assessed and optimized. It was shown that electrochromic metal-textile/PANI composites exhibit relatively stable electrochromic behaviour, retaining approximately half of its initial colour change performance after 100 switching cycles, indicating that a different electrochromic material should be employed in order to achieve potential feasibility of such textile-integrated electrochromic systems for textile-embedded visual interfaces and/or smart clothing applications.
Acknowledgments
The authors sincerely thank Agne Spokaite for technical editing of the manuscript. This research is/was funded by the European Regional Development Fund according to the supported activity "Research Projects Implemented by World-class Researcher Groups" under Measure No. 01.2.2-LMT-K-718 and Lithuanian Research Council. Project "Smart membranes for electrochemical devices" No 09.3.3-LMT-K-718-01-0063.