Abstract
Bilayer coatings consisting of a  inner layer and a
inner layer and a  outer layer were made on copper substrates
outer layer were made on copper substrates  and the photoelectrochemical properties of the derived bilayered photoelectrodes for corrosion protection application was investigated and compared with the performance of our previously studied
and the photoelectrochemical properties of the derived bilayered photoelectrodes for corrosion protection application was investigated and compared with the performance of our previously studied  bilayered photoelectrodes coating for similar applications.
bilayered photoelectrodes coating for similar applications.  and
and  sols were used for preparing the coatings. Investigations on the charge storage capacity of the two different bilayered coatings revealed that the
sols were used for preparing the coatings. Investigations on the charge storage capacity of the two different bilayered coatings revealed that the  system is capable of storing higher charge at less negative applied potentials, i.e.,
system is capable of storing higher charge at less negative applied potentials, i.e.,  vs SCE, whereas, the
vs SCE, whereas, the  system stores higher charge at more negative applied potentials, i.e.,
system stores higher charge at more negative applied potentials, i.e.,  vs SCE.
vs SCE.
Export citation and abstract BibTeX RIS
Copper has been proposed as the ideal material for construction of canisters to be used for the disposal of spent nuclear fuels because of its outstanding corrosion resistance, ease of fabrication, abundant availability, and overall cost-effectiveness. For these reasons, extensive research has been pursued for assessment of suitability of copper for these same purposes and, therefore, corrosion protection of copper in the conditions prevailing underground becomes a matter of concern because the passive and protective copper oxide layer does not form when the oxygen potentials are very low. In this context, cathodic protection offered by  in the presence of ultraviolet (UV) light could be envisaged as appropriate. There have been substantial efforts to exploit the photocatalytic applications of nano
in the presence of ultraviolet (UV) light could be envisaged as appropriate. There have been substantial efforts to exploit the photocatalytic applications of nano  in all fields that it is predicted to become a part of every individual's life in a few years. One of the most promising applications that has stimulated considerable interest in the recent past is its use as a photoanode for corrosion protection of metal and steel substrates. The working principle of photoaided cathodic protection is that when a metal coated with a thin layer of
in all fields that it is predicted to become a part of every individual's life in a few years. One of the most promising applications that has stimulated considerable interest in the recent past is its use as a photoanode for corrosion protection of metal and steel substrates. The working principle of photoaided cathodic protection is that when a metal coated with a thin layer of  is exposed to UV irradiation,
is exposed to UV irradiation,  -hole pairs are created in the
-hole pairs are created in the  layer. The photogenerated electrons can be transferred to the metal substrate thereby making its electrode potential more negative than its corrosion potential. The main advantage of this method is that the photoanode, i.e.,
layer. The photogenerated electrons can be transferred to the metal substrate thereby making its electrode potential more negative than its corrosion potential. The main advantage of this method is that the photoanode, i.e.,  in this case, does not get consumed during the process of corrosion protection, unlike a sacrificial-type cathodic protection. However, in the case of the applicability of
in this case, does not get consumed during the process of corrosion protection, unlike a sacrificial-type cathodic protection. However, in the case of the applicability of  coatings for corrosion protection of copper under subterranean conditions, there would be no light available for such a purpose. But this problem is not a serious one as the containers would hold high-level nuclear waste and the gamma radiation being emitted from them could itself be visualized as an in situ source of UV illumination, provided suitable radiation converters are incorporated in the protective
coatings for corrosion protection of copper under subterranean conditions, there would be no light available for such a purpose. But this problem is not a serious one as the containers would hold high-level nuclear waste and the gamma radiation being emitted from them could itself be visualized as an in situ source of UV illumination, provided suitable radiation converters are incorporated in the protective  -based coating. The exact mechanism of corrosion protection offered by a
-based coating. The exact mechanism of corrosion protection offered by a  coating on metal/steel substrate in presence of UV illumination has been discussed in detail in Refs. 1–4.
coating on metal/steel substrate in presence of UV illumination has been discussed in detail in Refs. 1–4.
One of the inherent disadvantages of using a plain  coating for anticorrosion applications is that the photoeffect ceases to be operative once the UV illumination stops shining on the
coating for anticorrosion applications is that the photoeffect ceases to be operative once the UV illumination stops shining on the  -coated substrate. A possible method for solving this problem would be to couple another semiconductor like
-coated substrate. A possible method for solving this problem would be to couple another semiconductor like  whose conduction band (CB) level is lower than that of
whose conduction band (CB) level is lower than that of  5 so that the photogenerated electrons could be transferred from
5 so that the photogenerated electrons could be transferred from  to
to  and the coupled semiconductor, i.e.,
and the coupled semiconductor, i.e.,  , should be capable of storing and releasing the photogenerated electrons. Another candidate semiconductor that could be coupled with
, should be capable of storing and releasing the photogenerated electrons. Another candidate semiconductor that could be coupled with  would be nano
would be nano  . There are several reports on the use of
. There are several reports on the use of  electrodes for electrochromic windows, where the reversibility for lithium insertion in
electrodes for electrochromic windows, where the reversibility for lithium insertion in  was used for transmissive electrochromic devices.6, 7 In addition, a recent report describes the application of hierarchically mesostructured
was used for transmissive electrochromic devices.6, 7 In addition, a recent report describes the application of hierarchically mesostructured  for use in solar cells,8 where the active component is nanometric ceria using a constructed organic-dye-free solar cell. The reason for this is that the bandgap of nanometric ceria was found to have shifted by
for use in solar cells,8 where the active component is nanometric ceria using a constructed organic-dye-free solar cell. The reason for this is that the bandgap of nanometric ceria was found to have shifted by  when compared to that of
when compared to that of  . In view of the potential applications of nanoceria, the present investigation was carried out with an objective to measure the photoelectrochemical properties of
. In view of the potential applications of nanoceria, the present investigation was carried out with an objective to measure the photoelectrochemical properties of  bilayer electrodes on copper substrates and to find out if such electrodes possess the desirable properties of an efficient photoanode for corrosion protection. The results obtained are compared with the performance of the
bilayer electrodes on copper substrates and to find out if such electrodes possess the desirable properties of an efficient photoanode for corrosion protection. The results obtained are compared with the performance of the  electrode for the same application.
electrode for the same application.
Experimental
Synthesis
A commercial 
 sol solution (pH 7.0) supplied by Nihon Parkerizing Co., Ltd., Japan, was used as the source of
sol solution (pH 7.0) supplied by Nihon Parkerizing Co., Ltd., Japan, was used as the source of  . The average crystallite size of
. The average crystallite size of  in the solution was
in the solution was  . Ceria nanoparticles were synthesized using a low energy wet chemical synthesis technique known as microemulsion, the details of which are given as follows. The microemulsion system consists of surfactant sodium bis(2-ethylhexyl)-sulfosuccinate (AOT), toluene, and Millipore DI water. All the chemicals were purchased from (Aldrich, Inc.). AOT was dissolved in toluene and
. Ceria nanoparticles were synthesized using a low energy wet chemical synthesis technique known as microemulsion, the details of which are given as follows. The microemulsion system consists of surfactant sodium bis(2-ethylhexyl)-sulfosuccinate (AOT), toluene, and Millipore DI water. All the chemicals were purchased from (Aldrich, Inc.). AOT was dissolved in toluene and  aqueous cerium nitrate solution was added. The mixture was stirred, and hydrogen peroxide was then added dropwise. The detailed synthesis procedure is explained in Ref. 9. The reaction was carried out for
aqueous cerium nitrate solution was added. The mixture was stirred, and hydrogen peroxide was then added dropwise. The detailed synthesis procedure is explained in Ref. 9. The reaction was carried out for  and then the reaction mixture was allowed to separate into two layers. The upper, pale yellow layer was toluene-containing nonagglomerated ceria nanoparticles and the lower layer was the aqueous phase. The morphology of
and then the reaction mixture was allowed to separate into two layers. The upper, pale yellow layer was toluene-containing nonagglomerated ceria nanoparticles and the lower layer was the aqueous phase. The morphology of  nanoparticles was studied by high resolution transmission electron microscopy (HRTEM) using a Philips HRTEM operated at
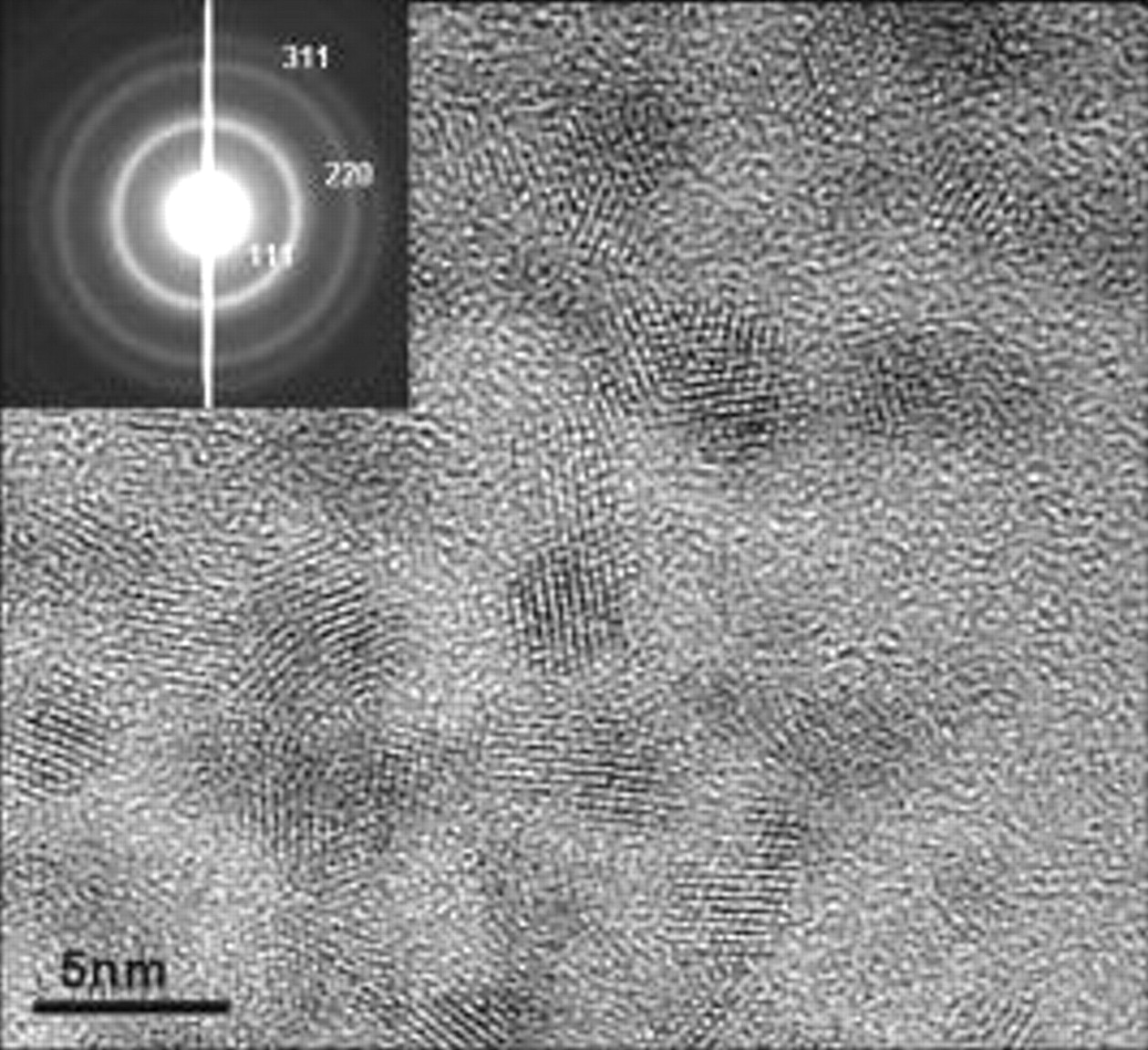
nanoparticles was studied by high resolution transmission electron microscopy (HRTEM) using a Philips HRTEM operated at  accelerating voltage. Figure 1 shows the HRTEM image of the synthesized cerium oxide nanoparticles.
accelerating voltage. Figure 1 shows the HRTEM image of the synthesized cerium oxide nanoparticles.
Figure 1. HRTEM image of nanoceria particles (size  ). (Inset shows the SAED pattern indicating the fluorite structure of nanoceria sample.)
). (Inset shows the SAED pattern indicating the fluorite structure of nanoceria sample.)
Copper specimens of dimensions  were used as the substrates. The copper plates were polished to a mirror finish with
were used as the substrates. The copper plates were polished to a mirror finish with 
 prior to coating and thoroughly degreased in acetone for
prior to coating and thoroughly degreased in acetone for  in an ultrasonic cleaner, which were subsequently used as substrates for coating the photoanode material. The photoanode coating consisted of two layers, the first one being
in an ultrasonic cleaner, which were subsequently used as substrates for coating the photoanode material. The photoanode coating consisted of two layers, the first one being  and the outer layer
and the outer layer  .
.  coatings on copper coupons were made using a dip coater, and the coating thickness was calculated using the weight difference of coupons before and after coating. The coating thickness was approximately
coatings on copper coupons were made using a dip coater, and the coating thickness was calculated using the weight difference of coupons before and after coating. The coating thickness was approximately  on each sample.
on each sample.  coatings on the
coatings on the  layer were made by the spin-coating technique in order to achieve a thickness of
layer were made by the spin-coating technique in order to achieve a thickness of  for the
for the  layer. The total thickness of the photoanode was measured to be approximately
layer. The total thickness of the photoanode was measured to be approximately  . The bilayered photoelectrode coating on the copper substrate was co-fired at
. The bilayered photoelectrode coating on the copper substrate was co-fired at  for
for  in a
in a  atmosphere to obviate oxidation of copper during heating. The heat treated coupns were subsequently subjected to photoelectrochemical characterization.
atmosphere to obviate oxidation of copper during heating. The heat treated coupns were subsequently subjected to photoelectrochemical characterization.
Photoelectrochemical characterization
Electrochemical characterization was carried out by measuring the open circuit potential (OCP) of the samples under dark and UV illuminated conditions with reference to a saturated calomel electrode (SCE). A deaerated 0.3% NaCl solution was used as the electrolyte unless otherwise mentioned, and a Pt strip was used as the counter electrode for polarization measurements. In order to simulate the conditions prevailing underground, a deaerated atmosphere was created during the electrochemical studies by purging the electrolyte solution with  (purity, 99.9%). A
(purity, 99.9%). A  Hg lamp was used as the source of UV illumination. The light first passed through an optical filter (UV-34, Kenko), which mostly
Hg lamp was used as the source of UV illumination. The light first passed through an optical filter (UV-34, Kenko), which mostly  allowed only light of wavelengths greater than
allowed only light of wavelengths greater than  . After this light passed through a quartz window, it was then made to fall on the coated side of the copper plate. A transparent electrochemical cell made of acryl fitted with a quartz window on one side was used. The sample was fixed on the opposite side with the aid of an O ring. A more detailed description of the experimental details is given in Ref. 10.
. After this light passed through a quartz window, it was then made to fall on the coated side of the copper plate. A transparent electrochemical cell made of acryl fitted with a quartz window on one side was used. The sample was fixed on the opposite side with the aid of an O ring. A more detailed description of the experimental details is given in Ref. 10.
For impedance measurements, the same conditions with respect to atmosphere and electrolyte as used for the OCP measurements were maintained. Impedance was measured as the current response to a superimposed ac signal of amplitude  with a frequency ranging from
with a frequency ranging from  up to
up to  at various applied potentials provided by a potentiostat, model 2090 HS supplied by Toho Techniques Research, Japan. After a preliminary analysis of the impedance data obtained for various applied potentials as a function of frequency, it was found that at lower frequencies
at various applied potentials provided by a potentiostat, model 2090 HS supplied by Toho Techniques Research, Japan. After a preliminary analysis of the impedance data obtained for various applied potentials as a function of frequency, it was found that at lower frequencies  , the system showed a capacitive behavior, and this was ascertained to be arising from the bilayer electrode coating. Hence, for measurement of the capacitance of the coating, the impedance was measured at potentials ranging from
, the system showed a capacitive behavior, and this was ascertained to be arising from the bilayer electrode coating. Hence, for measurement of the capacitance of the coating, the impedance was measured at potentials ranging from  down to
down to  in steps of
in steps of  at a low frequency of
at a low frequency of  . The system was kept for
. The system was kept for  at any applied potential before measurement, as it was verified that a constant current state was reached at
at any applied potential before measurement, as it was verified that a constant current state was reached at  after application of potential.
after application of potential.
Results and Discussion
As a first step, the response of the OCP of different samples to UV illumination was studied and their time evolution observed after exposure to UV source and after cutting it off.
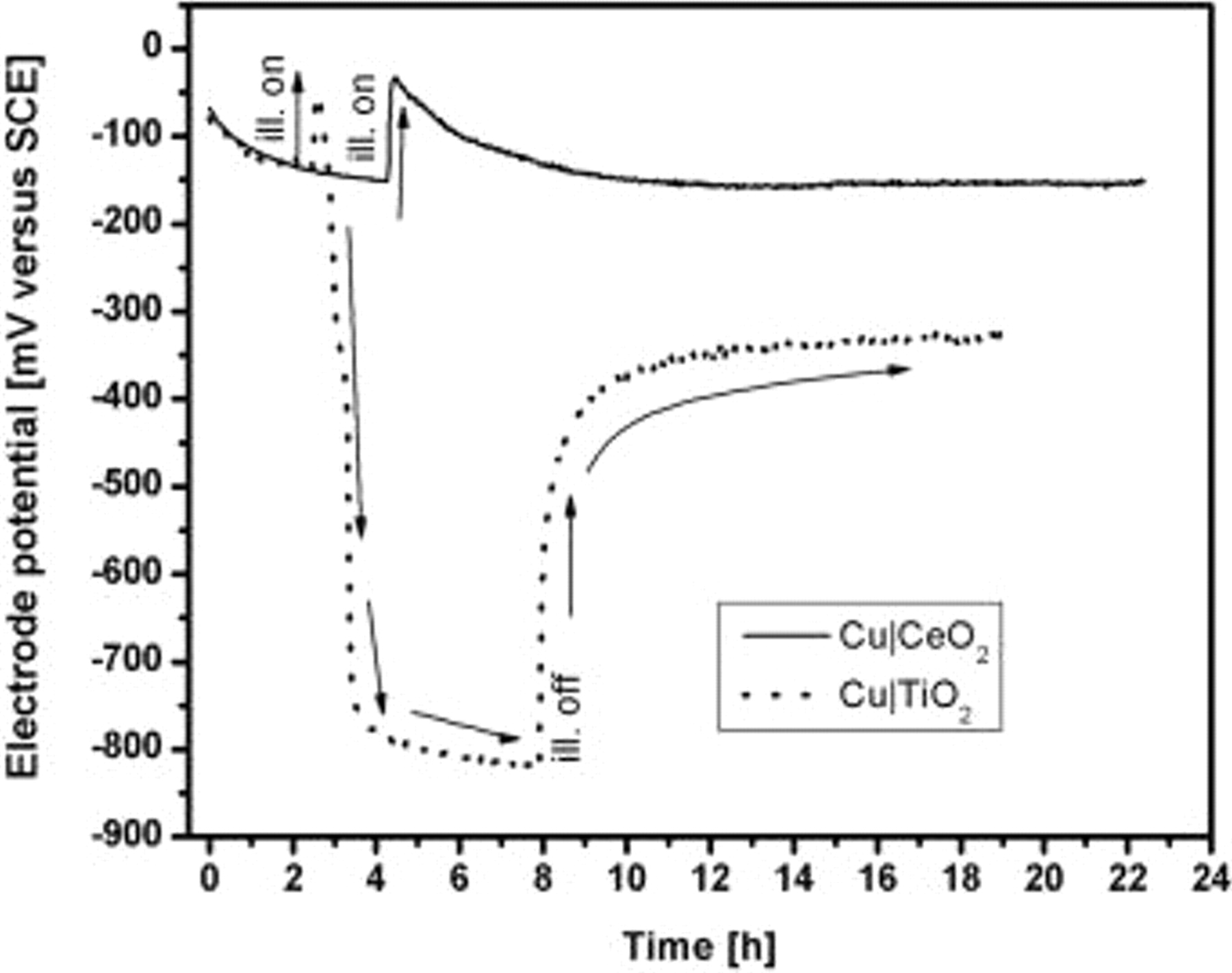
Figure 2 shows the time evolution of the OCP on exposure to UV illumination for  . It can be seen that there is an initial p-type effect (OCPs instantaneously becoming more positive) on exposure to UV light. But after sometime, the electrode potential slowly reverts back to negative values. Nevertheless, the values do not become more negative than
. It can be seen that there is an initial p-type effect (OCPs instantaneously becoming more positive) on exposure to UV light. But after sometime, the electrode potential slowly reverts back to negative values. Nevertheless, the values do not become more negative than  whereas, in the case of a plain
whereas, in the case of a plain  coating (also included in Fig. 2), though the same initial p-type effect is observed, after some time, the electrode potentials slowly become more negative and finally reach values of
coating (also included in Fig. 2), though the same initial p-type effect is observed, after some time, the electrode potentials slowly become more negative and finally reach values of  , which is the photopotential of
, which is the photopotential of  . The initial p-type effect is due to the presence of a thin layer of copper oxide
. The initial p-type effect is due to the presence of a thin layer of copper oxide  present between the copper and the semiconductor oxide coating, that could have formed due to the interaction of the copper surface with the water-based sol solution during heat-treatment.
present between the copper and the semiconductor oxide coating, that could have formed due to the interaction of the copper surface with the water-based sol solution during heat-treatment.  and CuO are both p-type semiconductors with bandgap equal to 2 and
and CuO are both p-type semiconductors with bandgap equal to 2 and  , respectively, and their presence adversely affects the normal n-type photoeffect in
, respectively, and their presence adversely affects the normal n-type photoeffect in  as shown in Fig. 2. The copper oxide layer can be reduced by photogenerated electrons from
as shown in Fig. 2. The copper oxide layer can be reduced by photogenerated electrons from  ,10 but the photoeffect in
,10 but the photoeffect in  , though capable of reducing the copper oxide layer, cannot make the OCP reach large negative values like
, though capable of reducing the copper oxide layer, cannot make the OCP reach large negative values like  (cf. Fig. 2). Hence, there is a necessity to include
(cf. Fig. 2). Hence, there is a necessity to include  in the photoanode containing
in the photoanode containing  . But since the electrical conductivity of
. But since the electrical conductivity of  is greater than that of
is greater than that of  , the design of a bilayer coating was thought of, where the photoeffect could be generated by an outer
, the design of a bilayer coating was thought of, where the photoeffect could be generated by an outer  layer, from which the photogenerated electrons could be passed on to the substrate via an intermediate
layer, from which the photogenerated electrons could be passed on to the substrate via an intermediate  layer, whose electrical conductivity was substantial to facilitate charge transfer. In addition, since
layer, whose electrical conductivity was substantial to facilitate charge transfer. In addition, since  is also capable of exhibiting multiple valence, it is expected to store charge in the form of photogenerated electrons according to Eq. 1, which will sustain the photoeffect even after cessation of UV illumination, by releasing the stored electrons to the substrate
is also capable of exhibiting multiple valence, it is expected to store charge in the form of photogenerated electrons according to Eq. 1, which will sustain the photoeffect even after cessation of UV illumination, by releasing the stored electrons to the substrate
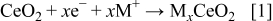
where  or Li and
or Li and  .
.
Figure 2. Comparison of the response of electrode potentials to UV illumination for  and
and  .
.
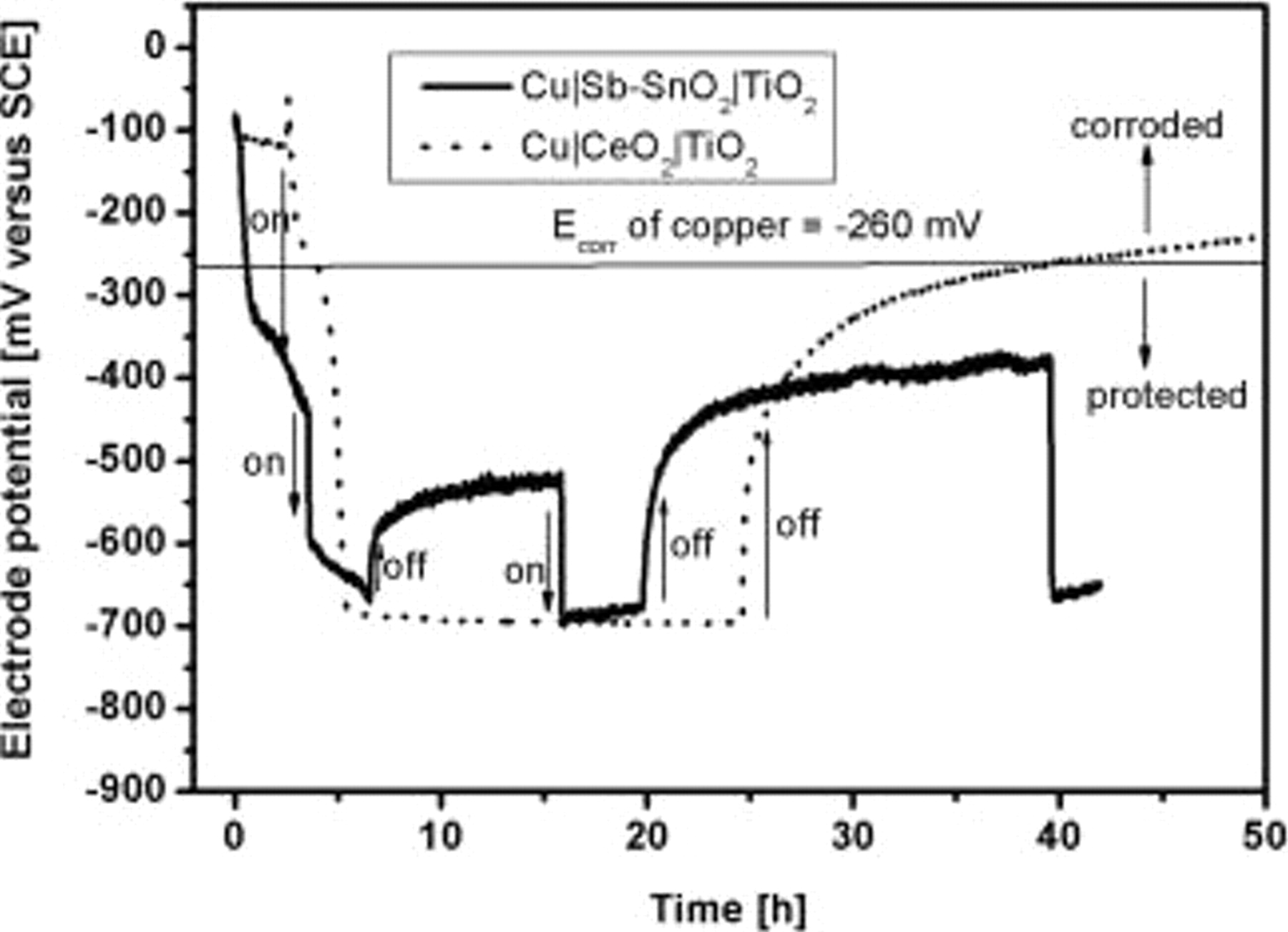
Figure 3 depicts a comparison of the response of the OCP response on exposure to UV illumination for the  which is also compared with that of
which is also compared with that of  (taken from Ref. 10). It can be seen that, though both the profiles are comparable with respect to the absolute values of the photopotentials, the recovery of the electrode potentials after the source of UV illumination has been cut off, differs to a slight extent. The electrode potential after the stopping of illumination is more negative for the bilayer electrode containing
(taken from Ref. 10). It can be seen that, though both the profiles are comparable with respect to the absolute values of the photopotentials, the recovery of the electrode potentials after the source of UV illumination has been cut off, differs to a slight extent. The electrode potential after the stopping of illumination is more negative for the bilayer electrode containing  than that obtained in the case of
than that obtained in the case of  and remains at such values for a longer period of time. This clearly indicates that the charge-storage property of
and remains at such values for a longer period of time. This clearly indicates that the charge-storage property of  is different from that of
is different from that of  , which result is expected. The charge-storage property could be quantified by measuring the capacitance as a function of applied voltage, which was carried out by measuring the impedance at low frequencies such as
, which result is expected. The charge-storage property could be quantified by measuring the capacitance as a function of applied voltage, which was carried out by measuring the impedance at low frequencies such as  , with varying applied potentials.
, with varying applied potentials.
Figure 3. Time evolution of electrode potential and its response to UV illumination compared for  and
and  .
.
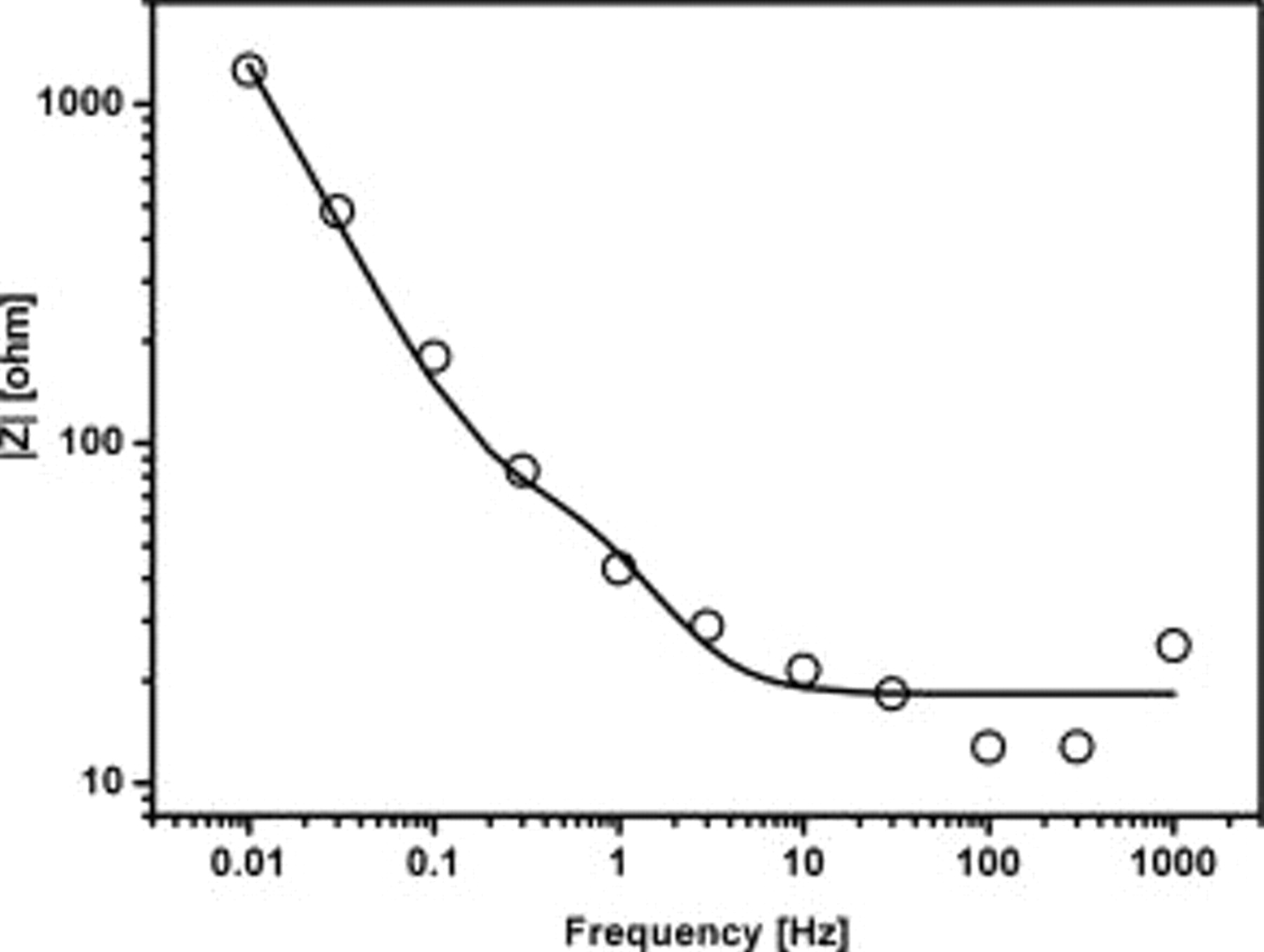
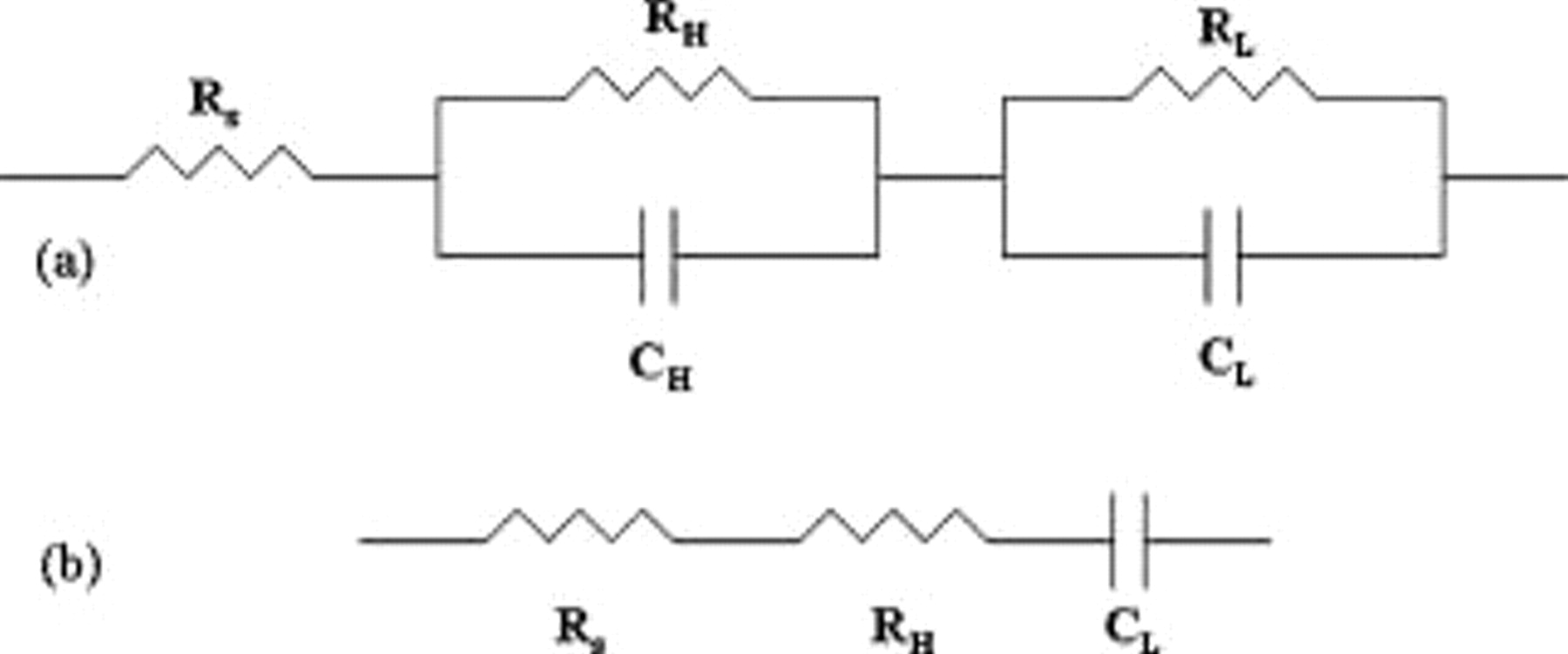
Figure 4 shows the Bode plot for the sample polarized at  vs SCE. An equivalent circuit corresponding to the configuration given in Fig. 5a was assumed for the analysis of the impedance data. The impedance information can be divided into three distinct regions.
vs SCE. An equivalent circuit corresponding to the configuration given in Fig. 5a was assumed for the analysis of the impedance data. The impedance information can be divided into three distinct regions.  represents the solution resistance (appears at very high frequencies);
represents the solution resistance (appears at very high frequencies);  are those from the high frequency response (due to electrode solution interface);
are those from the high frequency response (due to electrode solution interface);  are the resistance and capacitance components of the low frequency response (due to the semiconductor oxide coating). The value of each component was determined from the absolute value of the impedance determined at specific frequencies where only one of the components would be a major contributor to the total impedance, neglecting the effect due to others. The calculated data assuming the equivalent circuit fitted well with the measured data and is shown as a solid line in Fig. 4.
are the resistance and capacitance components of the low frequency response (due to the semiconductor oxide coating). The value of each component was determined from the absolute value of the impedance determined at specific frequencies where only one of the components would be a major contributor to the total impedance, neglecting the effect due to others. The calculated data assuming the equivalent circuit fitted well with the measured data and is shown as a solid line in Fig. 4.
Figure 4. Impedance measured as a function of frequency for the  HT Cu∣bilayered electrode at an applied potential of
HT Cu∣bilayered electrode at an applied potential of  vs SCE; solid line is the curve fitting according to equivalent circuit given in Fig. 5a.
vs SCE; solid line is the curve fitting according to equivalent circuit given in Fig. 5a.
Figure 5. (a) Equivalent circuit proposed conforming to the measured impedance data and (b) reduced circuit at low frequencies.
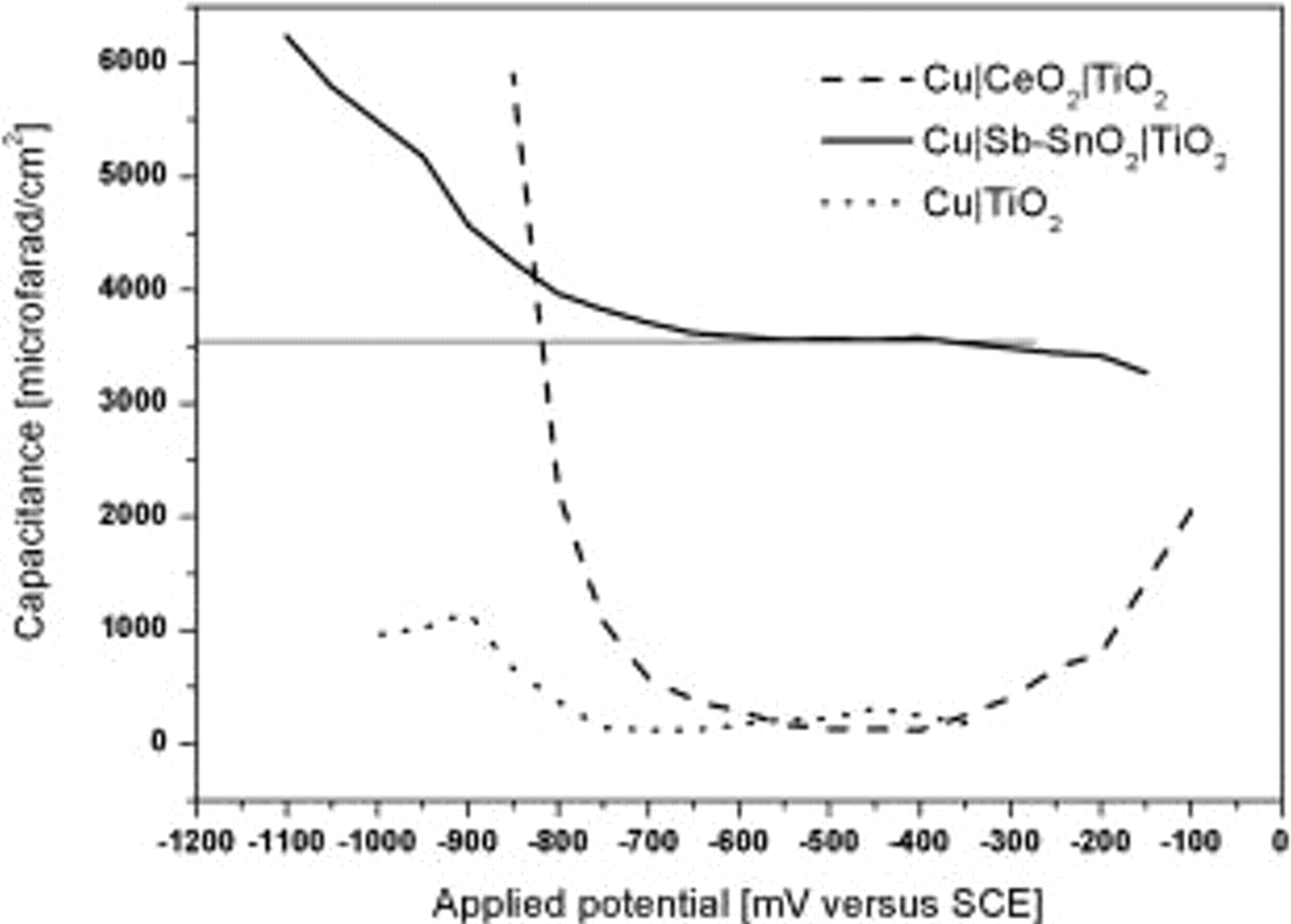
From the impedance measurements at  for various applied potentials, the capacitance of the bilayer coating was determined at each applied potential at this frequency assuming the equivalent circuit shown in Fig. 5b and plotted in Fig. 6. Figure 6 shows a comparison of the capacitance for
for various applied potentials, the capacitance of the bilayer coating was determined at each applied potential at this frequency assuming the equivalent circuit shown in Fig. 5b and plotted in Fig. 6. Figure 6 shows a comparison of the capacitance for  ,
,  , and
, and  samples measured as a function of the applied electrode potential vs SCE. The
samples measured as a function of the applied electrode potential vs SCE. The  electrode obviously stores less charge throughout the range of applied potentials of measurement when compared to the other two electrodes. This is due to the fast charge recombination (electron-hole) on the
electrode obviously stores less charge throughout the range of applied potentials of measurement when compared to the other two electrodes. This is due to the fast charge recombination (electron-hole) on the  surface. However, when one compares the charge-storage property of
surface. However, when one compares the charge-storage property of  and
and  , it is seen that
, it is seen that  exhibits a constant charge, i.e.,
exhibits a constant charge, i.e.,  stored over potentials from
stored over potentials from  down to
down to  and then there is a sudden increase in the amount of charge stored for still lower potentials. The large values of capacitance at
and then there is a sudden increase in the amount of charge stored for still lower potentials. The large values of capacitance at  corresponds to the one electron reduction from
corresponds to the one electron reduction from  to
to  followed by subsequent reduction to
followed by subsequent reduction to  as indicated by the sudden increase in the capacitance beyond
as indicated by the sudden increase in the capacitance beyond  . The high capacitance values of
. The high capacitance values of  at
at  confirm that when the electrode potential reaches the value of
confirm that when the electrode potential reaches the value of  during illumination (photopotential), there is a charging up of the electrode. When the source of illumination is cut off, this stored charge is slowly discharged to the substrate such that a highly negative electrode potential of the substrate is still maintained.
during illumination (photopotential), there is a charging up of the electrode. When the source of illumination is cut off, this stored charge is slowly discharged to the substrate such that a highly negative electrode potential of the substrate is still maintained.
Figure 6. Capacitance as a function of applied potential for  and
and  .
.
In the case of  there is low charge ranging from
there is low charge ranging from  stored at potentials from
stored at potentials from  down to
down to  and then there is a sudden increase in the stored charge, which is even higher than that stored by the
and then there is a sudden increase in the stored charge, which is even higher than that stored by the  electrode at even potentials. This indicates that the reduction of
electrode at even potentials. This indicates that the reduction of  to
to  takes place at a more negative electrode potential when compared to
takes place at a more negative electrode potential when compared to  reduction.
reduction.
It is desirable that the reduction of the multivalent species ( or
or  ) in the photoanode should take place at its photopotential, so that the photogenerated electrons can be transferred from
) in the photoanode should take place at its photopotential, so that the photogenerated electrons can be transferred from  and can be stored simultaneously. In view of this requirement, from the present study, it can be inferred that the
and can be stored simultaneously. In view of this requirement, from the present study, it can be inferred that the  electrode is preferable as a photoanode when compared to
electrode is preferable as a photoanode when compared to  . However, the
. However, the  electrode could also be made more efficient, if the photopotentials can be made around
electrode could also be made more efficient, if the photopotentials can be made around  , where an in situ reduction of Ce takes place and the charge can be reversibly stored and released to the substrate, thereby sustaining the photoeffect.
, where an in situ reduction of Ce takes place and the charge can be reversibly stored and released to the substrate, thereby sustaining the photoeffect.
Conclusion
The present study was carried out to evaluate and compare the performance of  bilayered photoelectrode with
bilayered photoelectrode with  . The photoelectrochemical properties were characterized by measuring the electrode potential response to UV illumination and the charge-storage capacity by impedance measurements as a function of applied electrode potential. The investigations revealed that
. The photoelectrochemical properties were characterized by measuring the electrode potential response to UV illumination and the charge-storage capacity by impedance measurements as a function of applied electrode potential. The investigations revealed that  bilayered photoelectrode showed good charge-storage capacity at the photopotential of
bilayered photoelectrode showed good charge-storage capacity at the photopotential of  . Nevertheless, the results also showed that if the photopotential of the electrode could be made
. Nevertheless, the results also showed that if the photopotential of the electrode could be made  or lower,
or lower,  bilayered photoelectrode is also capable of very high charge storage.
bilayered photoelectrode is also capable of very high charge storage.
Acknowledgment
R.S. acknowledges the National Institute for Materials Science, Tsukuba, Japan, for financial support in the form of a fellowship.
National Institute for Materials Science assisted in meeting the publication costs of this article.