Abstract
The electronic structure of the electrochemically Li-ion deintercalated  system has been investigated intensively with compositional
system has been investigated intensively with compositional  value variation using soft X-ray absorption spectroscopy (XAS) for oxygen K-edge and Co
value variation using soft X-ray absorption spectroscopy (XAS) for oxygen K-edge and Co  -edge. To get reasonably good XAS spectra for the electrochemically Li-ion deintercalated
-edge. To get reasonably good XAS spectra for the electrochemically Li-ion deintercalated  system, we made binder-free
system, we made binder-free  film electrodes using the electrostatic spray deposition technique. The spectral changes of the O K-edge XAS for
film electrodes using the electrostatic spray deposition technique. The spectral changes of the O K-edge XAS for  during charging are relatively more dramatic than those of the Co
during charging are relatively more dramatic than those of the Co  -edge XAS. From the results of the spectral changes of the Co L-edge and O K-edge XAS between
-edge XAS. From the results of the spectral changes of the Co L-edge and O K-edge XAS between  and
and  during charging, it is concluded that the substitution of Al for Co ions in
during charging, it is concluded that the substitution of Al for Co ions in  induces more increased oxygen participation in the charge compensation process during charging. © 2002 The Electrochemical Society. All rights reserved.
induces more increased oxygen participation in the charge compensation process during charging. © 2002 The Electrochemical Society. All rights reserved.
Export citation and abstract BibTeX RIS
Numerous studies on the electronic structure of cathode materials for Li rechargeable batteries have been made extensively. X-ray absorption spectroscopy (XAS) has been used to examine the electronic and local structure of the cathode materials.1 2 3 4 The XAS study for the Li-ion intercalation-deintercalation has been mainly investigated from the viewpoint of metal ion. The absorption peak features of the metal K-edge XAS include useful structural information such as oxidation state of chemical species, their site symmetries, and covalent bond strength. Further, it is observed that the small pre-edge peak was used to infer the electronic structure of the central atom, since the transition is very sensitive to chemical environments, in spite of the electric dipole-forbidden transition. It has been reported that the Li-ion deintercalation in lithium transition metal oxide systems leads to the increase of average oxidation state of metal ion and the local structural distortion around the metal atom. However, the metal K-edge XAS could not give any direct information for participation of oxygen in the charge compensation process. Only indirect inference for the contribution of oxygen atoms was obtained from the peak intensity for electronic transition of 1s electron to 4p orbital with shakedown process.
Recently, Ceder et al. reported higher open-circuit voltage (above  ) both theoretically and experimentally by the partial substitution of nontransition metals like
) both theoretically and experimentally by the partial substitution of nontransition metals like  for
for  ions in the
ions in the  structure.5 In this case, it has been suggested that the electrochemical property of electron exchange is much associated with the participation of an oxygen 2p band, in addition to the charge compensation by the metal ion. It is very important to investigate the quantity of oxygen atom contribution on the charge compensation during the Li-ion intercalation-deintercalation process. Soft XAS technique is widely used for the quantitative study of electronic structures for various cobalt oxides.6
7
8
9
10
11
12 Intensive absorption peaks of Co
structure.5 In this case, it has been suggested that the electrochemical property of electron exchange is much associated with the participation of an oxygen 2p band, in addition to the charge compensation by the metal ion. It is very important to investigate the quantity of oxygen atom contribution on the charge compensation during the Li-ion intercalation-deintercalation process. Soft XAS technique is widely used for the quantitative study of electronic structures for various cobalt oxides.6
7
8
9
10
11
12 Intensive absorption peaks of Co  -edge XAS representing intense main
-edge XAS representing intense main  transition, unlike the weak pre-edge peak of
transition, unlike the weak pre-edge peak of  transition in Co K-edge XAS, leads to direct information on the unoccupied molecular level. The electric dipole-allowed
transition in Co K-edge XAS, leads to direct information on the unoccupied molecular level. The electric dipole-allowed  transition of oxygen K-edge XAS also provides direct probe of the oxygen charge state and Co-O bonding interaction, since the 2p orbitals of oxygen ligand are involved in bonding configuration with Co metal ions under octahedral symmetry. Electronic transition from oxygen 1s core electron to the unoccupied molecular level by hybridization of the Co 3d orbital with the oxygen 2p orbital are characteristic pre-edge features. Hence, application of this soft XAS technique to
transition of oxygen K-edge XAS also provides direct probe of the oxygen charge state and Co-O bonding interaction, since the 2p orbitals of oxygen ligand are involved in bonding configuration with Co metal ions under octahedral symmetry. Electronic transition from oxygen 1s core electron to the unoccupied molecular level by hybridization of the Co 3d orbital with the oxygen 2p orbital are characteristic pre-edge features. Hence, application of this soft XAS technique to  systems could be a useful opportunity that estimates the degree of oxygen contribution for charge compensation in the Li-ion intercalation-deintercalation process.
systems could be a useful opportunity that estimates the degree of oxygen contribution for charge compensation in the Li-ion intercalation-deintercalation process.
Despite all the advantages of soft XAS for the investigation of the electronic structure of cathode materials for Li rechargeable batteries, its application to electrochemically Li-ion deintercalated cathode materials has been limited because its composite electrode consists of lithium transition metal oxide and some additives, including organic binder and carbon, which could be a hindrance to getting reasonably good data. In the present study, we have made binder-free film electrodes without any co-additives using the electrostatic spray deposition (ESD) technique. This allows more improved interpretation of O K-edge spectra for electrochemically Li-ion deintercalated  systems. The electronic structures of the
systems. The electronic structures of the  system were investigated on the basis of Co
system were investigated on the basis of Co  and oxygen K-edge XAS studies, thereby giving a better understanding of the electronic structure of electrochemically Li-ion deintercalated
and oxygen K-edge XAS studies, thereby giving a better understanding of the electronic structure of electrochemically Li-ion deintercalated  systems.
systems.
Experimental
 film was prepared by the ESD method. The working principles of ESD have been described in the literature.13
14 The stoichiometric amount of lithium nitrate, aluminum nitrate, and cobalt nitrate with a cationic ratio of
film was prepared by the ESD method. The working principles of ESD have been described in the literature.13
14 The stoichiometric amount of lithium nitrate, aluminum nitrate, and cobalt nitrate with a cationic ratio of  was dissolved in absolute ethanol and mixed to obtain a homogeneous precursor solution. A high voltage between the nozzle and Pt foil substrate makes the precursor solution atomized at the orifice of the nozzle, generating a fine aerosol spray. The temperature of the substrate was kept at 300°C during the deposition. The precursor solution was pumped at 2 mL/h rate for 1 h through a nozzle placed above the substrate. The
was dissolved in absolute ethanol and mixed to obtain a homogeneous precursor solution. A high voltage between the nozzle and Pt foil substrate makes the precursor solution atomized at the orifice of the nozzle, generating a fine aerosol spray. The temperature of the substrate was kept at 300°C during the deposition. The precursor solution was pumped at 2 mL/h rate for 1 h through a nozzle placed above the substrate. The  precursor was deposited on the Pt substrate and annealed at 800°C in air for 30 min. The homogeneous uniphase of the
precursor was deposited on the Pt substrate and annealed at 800°C in air for 30 min. The homogeneous uniphase of the  film was identified with X-ray diffraction (XRD) analysis. In order to minimize interference from the diffraction peaks of the Pt substrate, a grazing angle scan was made in which the angle of the incident radiation with respect to the plane of the substrate was fixed at 5°.
film was identified with X-ray diffraction (XRD) analysis. In order to minimize interference from the diffraction peaks of the Pt substrate, a grazing angle scan was made in which the angle of the incident radiation with respect to the plane of the substrate was fixed at 5°.
The Li-ion electrochemical deintercalation-reintercalation process was performed as follows. A three-electrode electrochemical cell was employed for electrochemical measurements in which lithium foil was used for both reference and counter electrodes. The electrolyte was used with 1 M  in propylene carbonate (PC) solution. All the electrochemical experiments were carried out at room temperature in a glove box filled with purified argon gas. For XAS experiments, the cells were first charged to a desired value of deintercalated Li-ion content (
in propylene carbonate (PC) solution. All the electrochemical experiments were carried out at room temperature in a glove box filled with purified argon gas. For XAS experiments, the cells were first charged to a desired value of deintercalated Li-ion content ( value) at a C/5 rate and then relaxed for a day. The electrochemical cells were disassembled in an argon-filled glove box, and the
value) at a C/5 rate and then relaxed for a day. The electrochemical cells were disassembled in an argon-filled glove box, and the  electrodes were taken out from the cell. These electrodes were then washed with tetrahydrofuran (THF) and dried thoroughly in a vacuum. All XAS experiments were carried out in an inert atmosphere, except during the insertion in the experimental XAS chamber, where the samples are exposed to air (less than 1 min).
electrodes were taken out from the cell. These electrodes were then washed with tetrahydrofuran (THF) and dried thoroughly in a vacuum. All XAS experiments were carried out in an inert atmosphere, except during the insertion in the experimental XAS chamber, where the samples are exposed to air (less than 1 min).
The soft XAS measurements of the  were performed on the U7 beamline in a storage ring of 2.5 GeV with a ring current of 120-160 mA at Pohang Light Source (PLS), which is a third-generation synchrotron radiation source.15 The U7 beamline, which consists of 4.3 m long, 7 cm period undulator, and the variable-included angle plane-grating monochromator, provides highly brilliant and monochromatic linear-polarized soft X-ray for the high-resolution spectroscopy.16 The O K-edge and Co
were performed on the U7 beamline in a storage ring of 2.5 GeV with a ring current of 120-160 mA at Pohang Light Source (PLS), which is a third-generation synchrotron radiation source.15 The U7 beamline, which consists of 4.3 m long, 7 cm period undulator, and the variable-included angle plane-grating monochromator, provides highly brilliant and monochromatic linear-polarized soft X-ray for the high-resolution spectroscopy.16 The O K-edge and Co  -edge XAS data were taken in a total electron yield mode, recording the sample current. The measurements are surface sensitive since the mean probing depth of this method is approximately 50 Å.17
-edge XAS data were taken in a total electron yield mode, recording the sample current. The measurements are surface sensitive since the mean probing depth of this method is approximately 50 Å.17
The experimental spectra were normalized by a reference signal from Au mesh with 90% transmission. The energy calibrations for O K-edge and Co  -edge were made using the L-edge data of pure V and Co metal foils, respectively. According to the measured photon absorption spectra specified as the inner shell electron excitation for the Ar,
-edge were made using the L-edge data of pure V and Co metal foils, respectively. According to the measured photon absorption spectra specified as the inner shell electron excitation for the Ar,  and Ne gases, the energy-resolving power
and Ne gases, the energy-resolving power  in the entire measurement range was greater than 3000. The base pressure of the experiment chamber was in the
in the entire measurement range was greater than 3000. The base pressure of the experiment chamber was in the  range.
range.
Results and Discussion
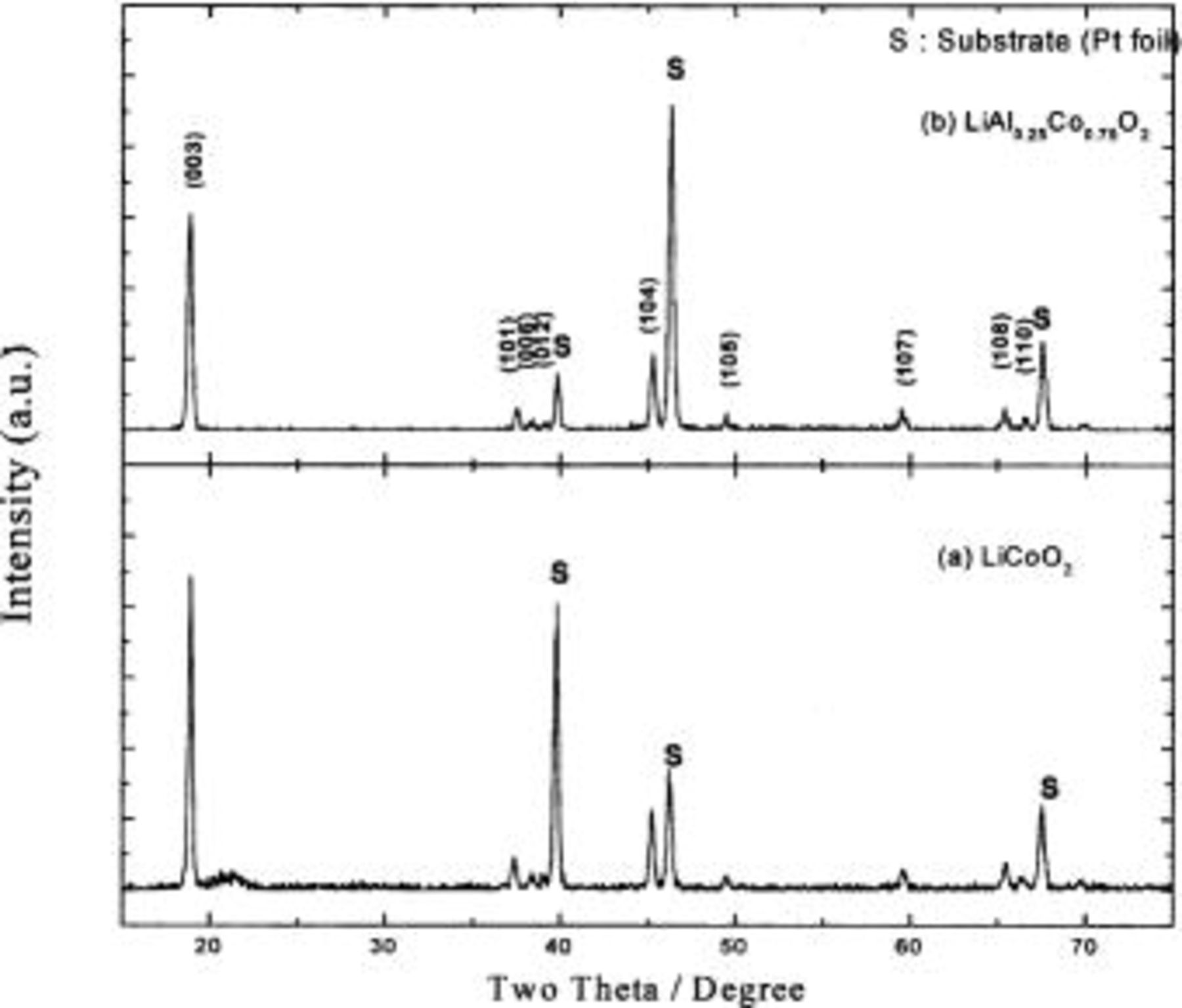
The  film was successfully deposited on Pt foil by ESD. Figure 1 shows XRD patterns of the
film was successfully deposited on Pt foil by ESD. Figure 1 shows XRD patterns of the  film annealed at 800°C for
film annealed at 800°C for  0 and (b) 0.25. As can be seen in Fig. 1, all diffraction peaks can be indexed using the hexagonal axes option for the rhombohedral
0 and (b) 0.25. As can be seen in Fig. 1, all diffraction peaks can be indexed using the hexagonal axes option for the rhombohedral  space group. The diffraction lines at the (006)/(012) and (108)/(110) couples for
space group. The diffraction lines at the (006)/(012) and (108)/(110) couples for  film annealed at 800°C show good splitting patterns, indicating that this sample has a well-developed, layered
film annealed at 800°C show good splitting patterns, indicating that this sample has a well-developed, layered  structure.18 The oxygen octahedra of the central Co atom are edge-shared within the octahedral layer and the Li atoms are placed in the lattice channel between interlayer planes. As in the case of
structure.18 The oxygen octahedra of the central Co atom are edge-shared within the octahedral layer and the Li atoms are placed in the lattice channel between interlayer planes. As in the case of  the XRD pattern of
the XRD pattern of  shows that it has the same
shows that it has the same  structure. The separations of the (006)/(012) and the (108)/(110) couples of diffraction lines of
structure. The separations of the (006)/(012) and the (108)/(110) couples of diffraction lines of  are wider than those of
are wider than those of  The further characterizations of this film electrode have been described in our previous work.19
20
The further characterizations of this film electrode have been described in our previous work.19
20
Figure 1. XRD patterns for  film annealed at 800°C for
film annealed at 800°C for  0 and (b) 0.25.
0 and (b) 0.25.
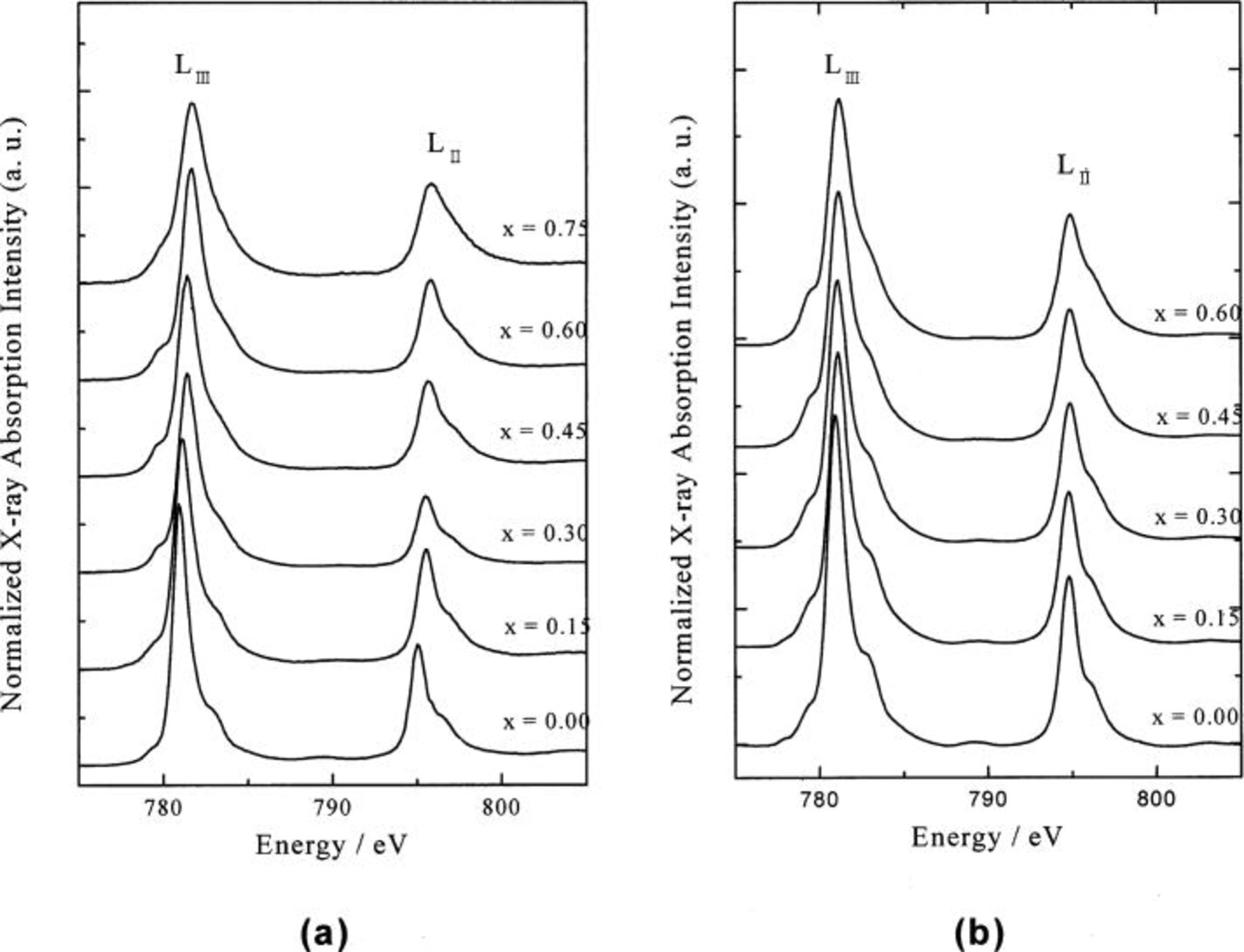
The electronic structures of Co ion in the  system can be investigated qualitatively with peak features in the present soft XAS study because the peak shapes and chemical shifts are very sensitive to the oxidation state, spin state, and bond covalency. Figure 2 shows the Co
system can be investigated qualitatively with peak features in the present soft XAS study because the peak shapes and chemical shifts are very sensitive to the oxidation state, spin state, and bond covalency. Figure 2 shows the Co  -edge XAS spectra of
-edge XAS spectra of  and
and  system with respect to the
system with respect to the  value. Respectively, the
value. Respectively, the  and
and  edges corresponded to transitions from the Co
edges corresponded to transitions from the Co  and
and  core electrons, split by the spin-orbit interaction of the Co 2p core level, to an unoccupied 3d level highly hybridized with oxygen 2p orbital. For the
core electrons, split by the spin-orbit interaction of the Co 2p core level, to an unoccupied 3d level highly hybridized with oxygen 2p orbital. For the  state in the
state in the  the corresponding electronic final states represent Co
the corresponding electronic final states represent Co  where the
where the  represents the hole of Co 2p core level.
represents the hole of Co 2p core level.
Figure 2. Normalized Co  -edge XAS as a function of
-edge XAS as a function of  for (a)
for (a)  and (b)
and (b) 
The Co  -edge XAS of
-edge XAS of  film is very similar to that of bulk
film is very similar to that of bulk  compound reported earlier.11 This means that the
compound reported earlier.11 This means that the  film has been successfully prepared on the Pt substrate with the ESD method and the
film has been successfully prepared on the Pt substrate with the ESD method and the  ion is present with only low-spin configuration. The Co
ion is present with only low-spin configuration. The Co  -edge of
-edge of  shows main peaks at
shows main peaks at  and
and  and a weak shoulder peak at
and a weak shoulder peak at  and
and  respectively, due to Co 2p-3d electrostatic interaction and the crystal field effect of octahedral symmetry. As shown in Fig. 2, there is no substantial difference between the Co
respectively, due to Co 2p-3d electrostatic interaction and the crystal field effect of octahedral symmetry. As shown in Fig. 2, there is no substantial difference between the Co  -edge XAS spectra of
-edge XAS spectra of  system during charging compared to that of
system during charging compared to that of  However, the Co
However, the Co  -edge XAS of
-edge XAS of  for the electrochemical deintercalation have been changed effectively with the
for the electrochemical deintercalation have been changed effectively with the  value, even though it could be said that the changes are not appreciable. The peaks are broader and shifted toward the higher energy region with increasing Li-ion deintercalation.
value, even though it could be said that the changes are not appreciable. The peaks are broader and shifted toward the higher energy region with increasing Li-ion deintercalation.
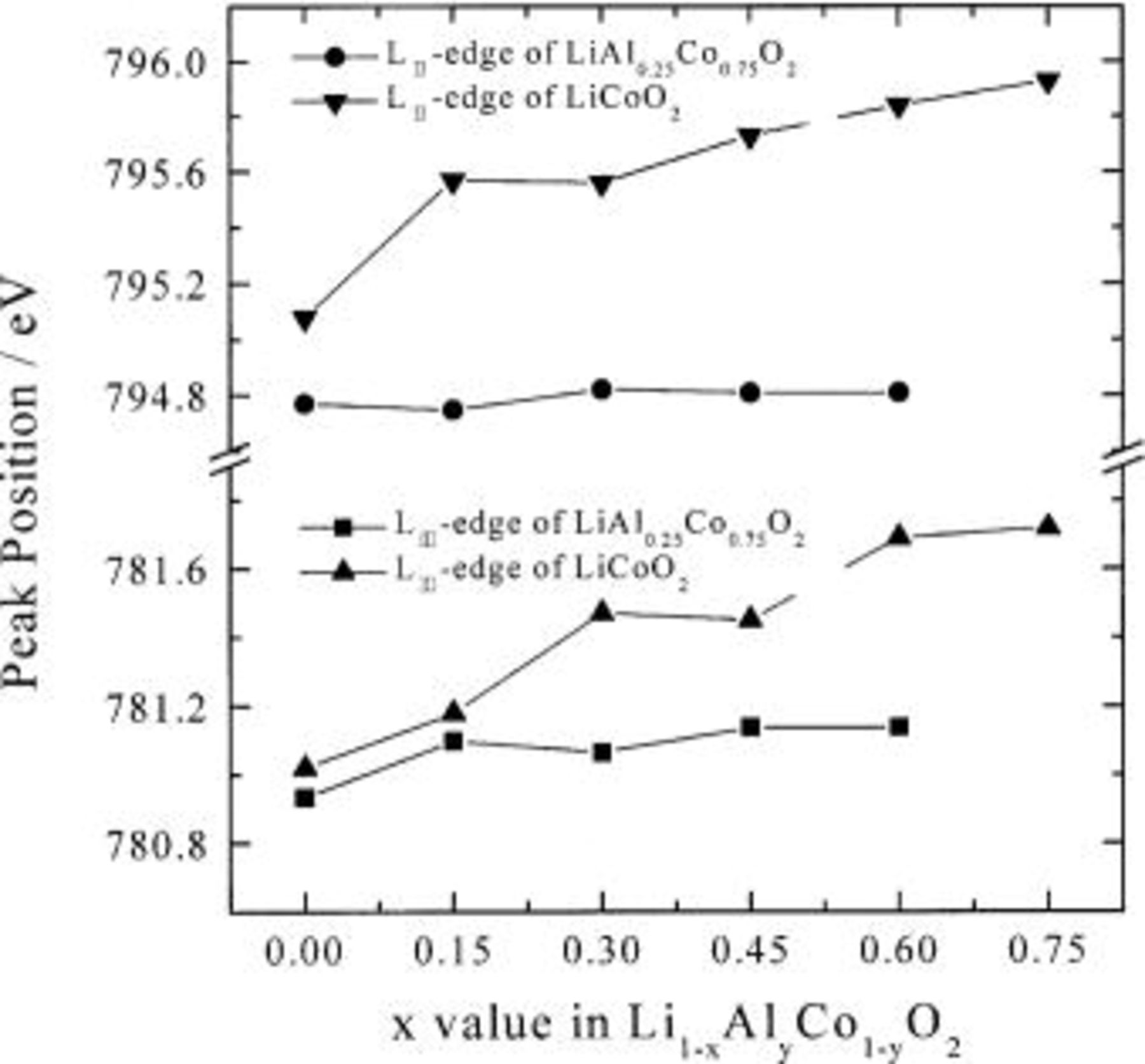
The relationship between the peak position and the deintercalated Li-ion content is depicted in Fig. 3. First, the peak position is proportional to the  value in
value in  The peak shift toward the higher energy region shows directly the partial evolution of
The peak shift toward the higher energy region shows directly the partial evolution of  ion with electrochemical deintercalation. It is reasonable that the higher absorption energy is necessary for Co ion under the higher oxidative environment in order to excite the 2p core electron, which is strongly bound to less screened nucleus. Actually, the electrochemical deintercalation leads to the existence of central atoms with different chemical bonding characters of
ion with electrochemical deintercalation. It is reasonable that the higher absorption energy is necessary for Co ion under the higher oxidative environment in order to excite the 2p core electron, which is strongly bound to less screened nucleus. Actually, the electrochemical deintercalation leads to the existence of central atoms with different chemical bonding characters of  and
and  bonds. In contrast, the Co
bonds. In contrast, the Co  -edge peak position of
-edge peak position of  is hardly changed during the electrochemical deintercalation. Considering the energy resolution
is hardly changed during the electrochemical deintercalation. Considering the energy resolution  the trivalent
the trivalent  low-spin state in
low-spin state in  remains mostly unaffected by Li deintercalation. As shown in Fig. 3, the substitution of Al for Co ions in
remains mostly unaffected by Li deintercalation. As shown in Fig. 3, the substitution of Al for Co ions in  reduces cobalt atom contribution on the charge compensation process during charging. This is confirmed by the following O K-edge XAS results.
reduces cobalt atom contribution on the charge compensation process during charging. This is confirmed by the following O K-edge XAS results.
Figure 3. Variations of peak position of Co  -edge XAS as a function of
-edge XAS as a function of  value.
value.
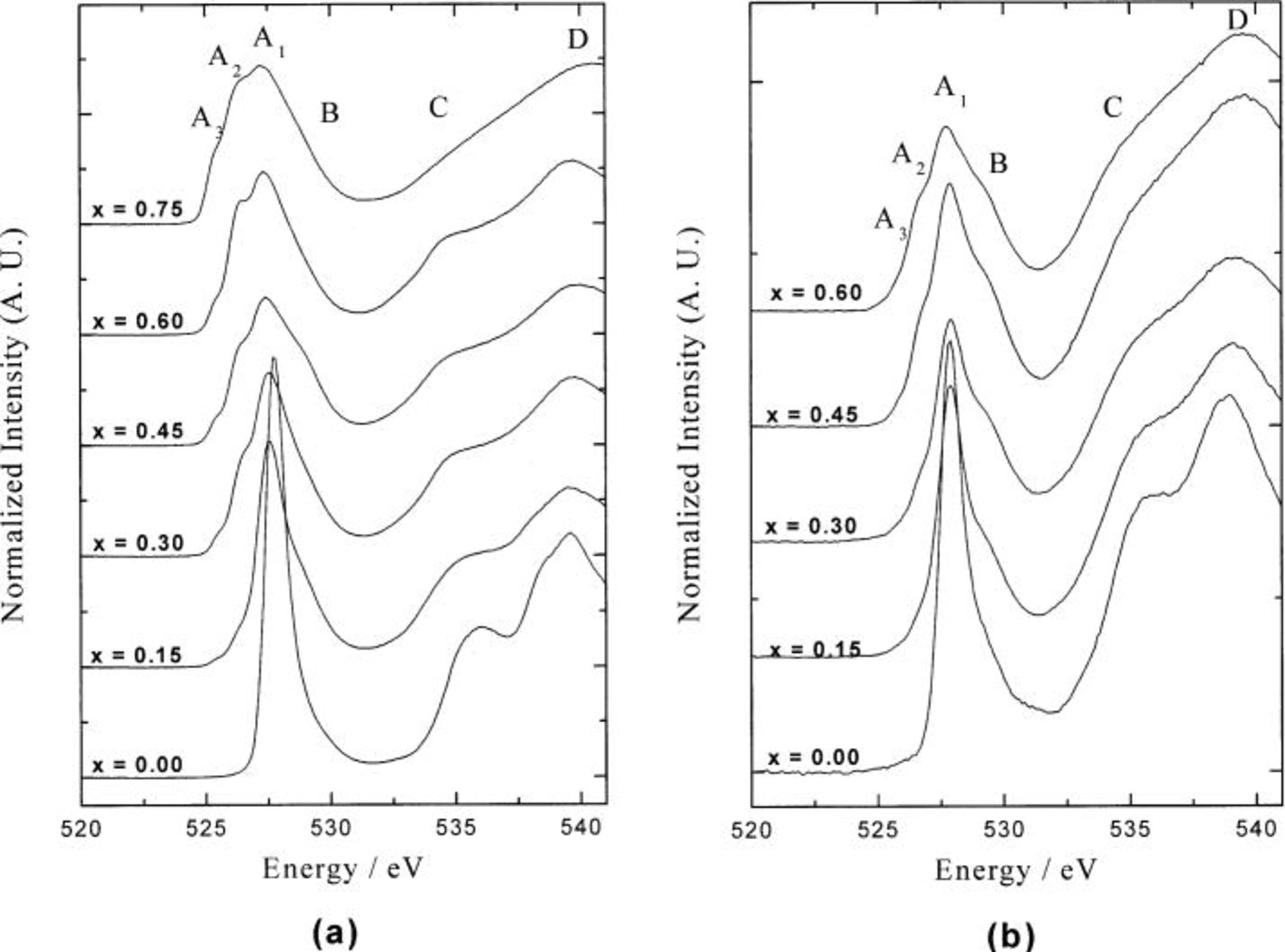
Figure 4 shows the O K-edge XAS of an electrochemically deintercalated Li-ion  system. The spectrum of pristine
system. The spectrum of pristine  presents a symmetric and intense absorption peak (
presents a symmetric and intense absorption peak ( peak) at
peak) at  and the broad, higher energy peaks (C and D peaks) above 536 eV. The first intense peak corresponds to the transition of oxygen 1s electron to the hybridized state of Co 3d and oxygen 2p orbitals, whereas the broad higher peaks correspond to the transitions to hybridized states of oxygen 2p and Co 4sp orbitals. In this case, the
and the broad, higher energy peaks (C and D peaks) above 536 eV. The first intense peak corresponds to the transition of oxygen 1s electron to the hybridized state of Co 3d and oxygen 2p orbitals, whereas the broad higher peaks correspond to the transitions to hybridized states of oxygen 2p and Co 4sp orbitals. In this case, the  peak corresponds to the transition to an unoccupied molecular level, including Co
peak corresponds to the transition to an unoccupied molecular level, including Co  -oxygen 2p character, since the oxygen 2p orbital is highly hybridized with 3d orbital of
-oxygen 2p character, since the oxygen 2p orbital is highly hybridized with 3d orbital of  ion with low spin
ion with low spin  electronic configuration under octahedral
electronic configuration under octahedral  symmetry. Although the oxygen
symmetry. Although the oxygen  3d transition is forbidden by the electric-dipole approximation, the appearance of the absorption peak is due to the hybridization of Co 3d and oxygen 2p orbitals. The
3d transition is forbidden by the electric-dipole approximation, the appearance of the absorption peak is due to the hybridization of Co 3d and oxygen 2p orbitals. The  peak corresponds to a final state of O
peak corresponds to a final state of O 
 electronic configuration, where
electronic configuration, where  is oxygen 1s core hole.
is oxygen 1s core hole.
Figure 4. Normalized oxygen K-edge XAS as a function of  for (a)
for (a)  and (b)
and (b) 
As a similar spectroscopic study with oxygen K-edge absorption, some research groups have built up the spectroscopic background of ligand K-edge absorption, applying sulfur and chlorine K-edge XAS to various inorganic materials.21
22
23
24
25 They have investigated the relationship between the pre-edge feature and the local structures such as ligand charge state and ligand-metal bond covalency. The ligand K-edge XAS gives important structural information that cannot be obtained from the metal K-edge XAS. The spectral changes of the O K-edge XAS are relatively more dramatic than those of the Co  -edge XAS with respect to the
-edge XAS with respect to the  value, and these changes indicate that the charge compensation for the electron exchange in the Li-ion deintercalation process can be achieved more in oxygen site than Co metal atomic site. The spectroscopic features give important information in which the Li-ion deintercalation has much larger influence on the electronic structure of oxygen ion rather than that of Co ion. As the degree of electrochemical deintercalation increases (the
value, and these changes indicate that the charge compensation for the electron exchange in the Li-ion deintercalation process can be achieved more in oxygen site than Co metal atomic site. The spectroscopic features give important information in which the Li-ion deintercalation has much larger influence on the electronic structure of oxygen ion rather than that of Co ion. As the degree of electrochemical deintercalation increases (the  value increases), the
value increases), the  peak intensity decreases gradually and an additional broad peak B evolves as a shoulder peak in the higher region of
peak intensity decreases gradually and an additional broad peak B evolves as a shoulder peak in the higher region of  A chemical shift in the ligand 1s core energy is related to the effective charge on the ligand. The greater the effective nuclear charge of the ligand, the more oxidative the ligand site shifts the ligand pre-edge peak position to the higher energy region, since the higher absorption energy is necessary for the more oxidized oxygen ion in order to excite the oxygen 1s core electron, which is strongly bound to less screened nucleus. Therefore, the shoulder absorption peak in the higher energy region than the threshold energy can be assigned to the higher oxidation of the oxygen site on Li deintercalation, which indicates that the charge compensation for the electron exchange in the Li-ion deintercalation process could be achieved in the oxygen site. In the case of
A chemical shift in the ligand 1s core energy is related to the effective charge on the ligand. The greater the effective nuclear charge of the ligand, the more oxidative the ligand site shifts the ligand pre-edge peak position to the higher energy region, since the higher absorption energy is necessary for the more oxidized oxygen ion in order to excite the oxygen 1s core electron, which is strongly bound to less screened nucleus. Therefore, the shoulder absorption peak in the higher energy region than the threshold energy can be assigned to the higher oxidation of the oxygen site on Li deintercalation, which indicates that the charge compensation for the electron exchange in the Li-ion deintercalation process could be achieved in the oxygen site. In the case of  the increase of B peak intensity with increasing
the increase of B peak intensity with increasing  is much larger than that of
is much larger than that of  which shows that the oxygen site in Al-doped
which shows that the oxygen site in Al-doped  shows a larger contribution to the charge compensation process during charging compared to
shows a larger contribution to the charge compensation process during charging compared to  According to the previous first-principles calculations of Ceder et al. , the substitution of nontransition metal ion like
According to the previous first-principles calculations of Ceder et al. , the substitution of nontransition metal ion like  for
for  ion induces more increased oxygen participation in the electron exchange, which leads to higher open-circuit voltage above
ion induces more increased oxygen participation in the electron exchange, which leads to higher open-circuit voltage above  Our experimental XAS results are in good agreement with their theoretical work.
Our experimental XAS results are in good agreement with their theoretical work.
Li-ion deintercalation also gives rise to the gradual formation of two additional well-resolved absorption peaks in the lower energy region than the threshold energy. The peak intensities increase systematically with Li-ion deintercalation. Based on the earlier reports of the ligand K-edge absorption,21
22
23
24
25 the ligand pre-edge peak position shifts to the lower energy region due to both the local structural distortion and the increased effective nuclear charge of metal ion. The  peak intensity decreases with
peak intensity decreases with  value, while the relative intensities of the
value, while the relative intensities of the  and
and  peaks increase. The variation of the peak intensity with the electrochemical deintercalation can give important structural information about the hole state distribution and the effective charge on the oxygen atom, since the density of empty bound state in molecular energy level is related to the hybridization of Co 3d-O 2p orbitals. When the electronic structure of
peaks increase. The variation of the peak intensity with the electrochemical deintercalation can give important structural information about the hole state distribution and the effective charge on the oxygen atom, since the density of empty bound state in molecular energy level is related to the hybridization of Co 3d-O 2p orbitals. When the electronic structure of  ion is
ion is  the oxygen 1s core electron can be excited to new unoccupied molecular orbitals of one
the oxygen 1s core electron can be excited to new unoccupied molecular orbitals of one  and four
and four  states hybridized with oxygen 2p orbitals. Therefore, we can say that the
states hybridized with oxygen 2p orbitals. Therefore, we can say that the  and
and  peaks represent the final states of O
peaks represent the final states of O 
 and O
and O 
 electronic configurations, respectively. This is consistent with O K-edge results of a mixed valence
electronic configurations, respectively. This is consistent with O K-edge results of a mixed valence  system by Warda et al.8
system by Warda et al.8
As shown in Fig. 4, the change of  and
and  peak intensity in
peak intensity in  with increasing
with increasing  is smaller than that of
is smaller than that of  this indicates that the Co site in
this indicates that the Co site in  shows much smaller contribution to the charge compensation process during charging than in the case of
shows much smaller contribution to the charge compensation process during charging than in the case of  The results of Co L-edge and O K-edge analysis for the electrochemically Li-ion deintercalated
The results of Co L-edge and O K-edge analysis for the electrochemically Li-ion deintercalated  system indicate that the substitution of Al for Co ions in
system indicate that the substitution of Al for Co ions in  induces increased oxygen participation in the charge compensation process during charging.
induces increased oxygen participation in the charge compensation process during charging.
Conclusions
The  film has been successfully deposited on Pt foil by ESD. The electronic structure for the electrochemically Li-ion deintercalated
film has been successfully deposited on Pt foil by ESD. The electronic structure for the electrochemically Li-ion deintercalated  film has been investigated intensively with soft XAS at O K-edge and Co
film has been investigated intensively with soft XAS at O K-edge and Co  -edge. The results of Co L-edge XAS spectra during charging for
-edge. The results of Co L-edge XAS spectra during charging for  and
and  show that Al doping reduces cobalt participation in the charge compensation process during charging. The spectral changes of the O K-edge XAS for
show that Al doping reduces cobalt participation in the charge compensation process during charging. The spectral changes of the O K-edge XAS for  during charging are relatively more dramatic than those of the Co
during charging are relatively more dramatic than those of the Co  -edge XAS, and this indicates that the charge compensation for the electron exchange in the Li-ion deintercalation process can be achieved more in oxygen sites than Co metal atomic sites. From the comparison of the spectral changes of the Co L-edge and O K-edge XAS between
-edge XAS, and this indicates that the charge compensation for the electron exchange in the Li-ion deintercalation process can be achieved more in oxygen sites than Co metal atomic sites. From the comparison of the spectral changes of the Co L-edge and O K-edge XAS between  and
and  during charging, it becomes clear that the substitution of Al for Co ions in
during charging, it becomes clear that the substitution of Al for Co ions in  induces increased oxygen participation in the charge compensation process during charging.
induces increased oxygen participation in the charge compensation process during charging.
Acknowledgments
This work was supported by Brain Korea 21. The authors are grateful to authorities at the Pohang Light Source (PLS) for XAS measurements. This work was also supported in part by the Ministry of Information and Communication of Korea (Support Project of University Information Technology Research Center, supervised by KIPA).
Yonsei University assisted in meeting the publication costs of this article.