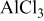Abstract
Electrolyte solutions for rechargeable Mg batteries were developed, based on reaction products of phenyl magnesium chloride (PhMgCl) Lewis base and  Lewis acid in ethers. The transmetallation of these ligands forms solutions with
Lewis acid in ethers. The transmetallation of these ligands forms solutions with  and
and  ions as the major ionic species, as analyzed by multinuclei nuclear magnetic resonance spectroscopy. Tetrahydrofuran (THF) solutions of
ions as the major ionic species, as analyzed by multinuclei nuclear magnetic resonance spectroscopy. Tetrahydrofuran (THF) solutions of  exhibit optimal properties: highly reversible Mg deposition (100% cycling efficiency) with low overvoltage:
exhibit optimal properties: highly reversible Mg deposition (100% cycling efficiency) with low overvoltage:  and electrochemical windows wider than
and electrochemical windows wider than  . A specific conductivity of
. A specific conductivity of  could be measured between
could be measured between  for these solutions, similar to that of standard electrolyte solutions for Li batteries. Mg ions intercalate reversibly with Chevrel phase
for these solutions, similar to that of standard electrolyte solutions for Li batteries. Mg ions intercalate reversibly with Chevrel phase  cathodes in these solutions. These systems exhibit high thermal stability. The solutions may enable the use of high voltage, high-capacity Mg insertion materials as cathodes and hence open the door for research and development of high-energy density, rechargeable Mg batteries.
cathodes in these solutions. These systems exhibit high thermal stability. The solutions may enable the use of high voltage, high-capacity Mg insertion materials as cathodes and hence open the door for research and development of high-energy density, rechargeable Mg batteries.
Export citation and abstract BibTeX RIS
There is an ongoing interest in nonaqueous magnesium electrochemistry. The thermodynamics and electrochemical behavior of Grignard reactions solutions (RMgX,  liquid,
liquid,  in ether solvents) was thoroughly studied.1–3 The first attempts to develop rechargeable Mg batteries and electrolyte solutions for such batteries were described in the early nineties.4, 5 It should be noted that in contrast to the case of Li electrodes, which may behave reversibly in a wide variety of polar aprotic Li salt solutions, while being covered by passivating surface films,6 Mg electrodes can behave reversibly only if they are bare and surface-film free.7 The difference between the two systems is because the surface films formed on lithium, which contain ionic lithium compounds (insoluble Li salts), are Li-ion conductors under an electrical field, while surface films comprising Mg salts are completely blocking and cannot transport the bivalent Mg ions. Mg electrodes are indeed bare in ether/RMgX (Grignard) solutions and hence behave fully reversibly in them.3 However, the electrochemical window of ether/RMgX solutions is too narrow (very low anodic stability), which prevents their use as electrolyte solutions for batteries.
in ether solvents) was thoroughly studied.1–3 The first attempts to develop rechargeable Mg batteries and electrolyte solutions for such batteries were described in the early nineties.4, 5 It should be noted that in contrast to the case of Li electrodes, which may behave reversibly in a wide variety of polar aprotic Li salt solutions, while being covered by passivating surface films,6 Mg electrodes can behave reversibly only if they are bare and surface-film free.7 The difference between the two systems is because the surface films formed on lithium, which contain ionic lithium compounds (insoluble Li salts), are Li-ion conductors under an electrical field, while surface films comprising Mg salts are completely blocking and cannot transport the bivalent Mg ions. Mg electrodes are indeed bare in ether/RMgX (Grignard) solutions and hence behave fully reversibly in them.3 However, the electrochemical window of ether/RMgX solutions is too narrow (very low anodic stability), which prevents their use as electrolyte solutions for batteries.
About eight years ago we demonstrated the possibility of elaborating rechargeable Mg battery systems based on a Mg metal anode, 
 Chevrel phase cathode and ethereal electrolyte solutions that contain complex electrolytes formed by reactions between
Chevrel phase cathode and ethereal electrolyte solutions that contain complex electrolytes formed by reactions between  Lewis base and
Lewis base and  Lewis acid (
Lewis acid ( , butylate).8 During the past few years, these complex solutions were studied thoroughly.9–12 It was found that their electrochemical properties, such as specific conductivity, reversibility of Mg deposition, and anodic stability, depends strongly on the Lewis acid–Lewis base ratio of the reagents, the identity of the organic ligands—R, and the concentration of the complex electrolyte in the solutions. For instance, tetrahydrofuran (THF) solutions of
, butylate).8 During the past few years, these complex solutions were studied thoroughly.9–12 It was found that their electrochemical properties, such as specific conductivity, reversibility of Mg deposition, and anodic stability, depends strongly on the Lewis acid–Lewis base ratio of the reagents, the identity of the organic ligands—R, and the concentration of the complex electrolyte in the solutions. For instance, tetrahydrofuran (THF) solutions of  and
and  at a ratio of 1:2 in concentrations between 0.2 and
at a ratio of 1:2 in concentrations between 0.2 and  , exhibit an electrochemical window of
, exhibit an electrochemical window of  , specific conductivity of a few
, specific conductivity of a few  and the 100% cycling efficiency and reversibility of magnesium deposition-dissolution processes. The redox activity of Chevrel phases (e.g.,
and the 100% cycling efficiency and reversibility of magnesium deposition-dissolution processes. The redox activity of Chevrel phases (e.g.,  ), which intercalate Mg ions reversibly, falls well within the electrochemical window of these solutions.
), which intercalate Mg ions reversibly, falls well within the electrochemical window of these solutions.
The present work is an attempt to develop electrolyte solutions with improved properties for all aspects of rechargeable Mg batteries. It was assumed that using complex salt solutions with phenyl instead of alkyl ligands might enable the extension of their electrochemical window and increase their specific conductivity, but not because of the reversibility of Mg deposition. Simple and commercially available reagents have been used, i.e.,  (PhMgCl) and
(PhMgCl) and  , as precursors. The solution's structure was analyzed by multinuclei nuclear magnetic resonance (NMR,
, as precursors. The solution's structure was analyzed by multinuclei nuclear magnetic resonance (NMR,  ,
,  ). Voltammetry and chronopotentiometry were applied for electrochemical studies. The morphology of Mg deposition was studied by scanning electron microscopy (SEM), and the thermal stability of the solutions, both as neat systems and with electrode materials (Mg,
). Voltammetry and chronopotentiometry were applied for electrochemical studies. The morphology of Mg deposition was studied by scanning electron microscopy (SEM), and the thermal stability of the solutions, both as neat systems and with electrode materials (Mg,  ), was studied by differential scanning calorimetry (DSC).
), was studied by differential scanning calorimetry (DSC).
Experimental
All chemical preparations and electrochemical measurements were carried out under pure argon atmosphere in M. Braun, Inc., glove boxes ( of water and oxygen). The preparation of "all phenyl" complex (APC) salt solutions involves a reaction between aluminum chloride (Aldrich, 99.99%) dissolved in THF (T.J. Backer
of water and oxygen). The preparation of "all phenyl" complex (APC) salt solutions involves a reaction between aluminum chloride (Aldrich, 99.99%) dissolved in THF (T.J. Backer  water) and phenyl magnesium chloride (Aldrich,
water) and phenyl magnesium chloride (Aldrich,  solution in THF). Different concentrations and molar ratios of reactants were examined in order to obtain optimal compositions. Each composition had different characteristics, such as electrochemical stability, overpotential for magnesium deposition, reversibility of magnesium deposition, ionic conductivity, etc.
solution in THF). Different concentrations and molar ratios of reactants were examined in order to obtain optimal compositions. Each composition had different characteristics, such as electrochemical stability, overpotential for magnesium deposition, reversibility of magnesium deposition, ionic conductivity, etc.
Nuclear magnetic resonance (NMR) spectra of the solutions were measured with a Bruker DMX-600 spectrometer at 156.3  and 36.72
and 36.72  MHz. Solutions of different concentrations and ratios of reagents in THF were examined in
MHz. Solutions of different concentrations and ratios of reagents in THF were examined in  NMR tubes without a deuterium lock.
NMR tubes without a deuterium lock.  and
and  chemical shifts are reported relative to external references: a solution of
chemical shifts are reported relative to external references: a solution of  in
in  with a drop of concentrated HCl
with a drop of concentrated HCl  and a saturated solution of
and a saturated solution of  in
in  , respectively. All the measurements were performed at room temperature
, respectively. All the measurements were performed at room temperature  .
.
Electrochemical measurements were performed with an EG&G PAR 273 potentiostat controlled by the Corrware program (Scribner, Inc.). Magnesium foils served both as the counter and reference electrodes in three-electrodes cells. Platinum wire electrodes were used for studying Mg deposition-dissolution processes and the anodic behavior of the solutions. The anodic limit of the solutions' electrochemical window was determined at the deflection points in their potentiodynamic response, where the  vs
vs  curves showed a rapid increase in the positive currents. Composite electrodes (cathodes for Mg batteries) comprising
curves showed a rapid increase in the positive currents. Composite electrodes (cathodes for Mg batteries) comprising  as the active mass (80–85%), polyvinyldifluorine (PVDF) binder (5–10%) and carbon black additive (5–10%) were also measured in the electrolyte solutions. The preparation of
as the active mass (80–85%), polyvinyldifluorine (PVDF) binder (5–10%) and carbon black additive (5–10%) were also measured in the electrolyte solutions. The preparation of  was described in detail elsewhere.
was described in detail elsewhere.
Prototype rechargeable Mg batteries comprising Mg foil anode ( thick),
thick),  cathode and separator (woven glass or porous polypropylene) soaked with electrolyte solution, were tested in coin type cells (standard 2030 parts from NRC Canada). A computerized multichannel electrochemical analyzer from Arbin, Inc. (model BT2000) was used for this purpose. Specific conductivities at different concentrations, reactants molar ratios, and temperature were measured by a HIOKI 3560 conductometer at
cathode and separator (woven glass or porous polypropylene) soaked with electrolyte solution, were tested in coin type cells (standard 2030 parts from NRC Canada). A computerized multichannel electrochemical analyzer from Arbin, Inc. (model BT2000) was used for this purpose. Specific conductivities at different concentrations, reactants molar ratios, and temperature were measured by a HIOKI 3560 conductometer at  .
.
Thermal studies were carried out using differential scanning calorimetry (DSC) using a DSC system from Mettler Toledo, Inc. (model DSC822). Solution samples alone usually  ), solutions in contact with Mg disks (
), solutions in contact with Mg disks ( diam,
diam,  ), with
), with  , with
, with  (prepared by chemical magnesiation by
(prepared by chemical magnesiation by  ) and solutions in contact with both Mg and
) and solutions in contact with both Mg and  together, were measured by DSC, sealed hermetically in high-pressure gold-plated stainless crucibles,
together, were measured by DSC, sealed hermetically in high-pressure gold-plated stainless crucibles,  in volume. The samples were heated at
in volume. The samples were heated at  , heating/cooling rate of
, heating/cooling rate of  .
.
A JEOL JSM-840 scanning electron microscope (SEM) with X-ray microanalysis spectroscopy (EDAX) was used to examine the surface morphology and the elemental composition of electrodeposited magnesium on copper foils. The samples for SEM measurements were washed in a glove box with pure THF before the measurements and were transferred from the glove box to the microscope using a special transfer system, which is described elsewhere.
All the above measurements with the electrolyte solutions were compared to reference measurements with electrolyte solutions comprising 
 in THF, which preparation and studies were described elsewere.
in THF, which preparation and studies were described elsewere.
Results and Discussion
Basic electrochemical performance
It was concluded from previous studies of ethereal solutions of  complexes that transmetallation of the ligands leads to the formation of species, such as
complexes that transmetallation of the ligands leads to the formation of species, such as  or
or  cations, and
cations, and  anions, which determine the electrochemical properties of these solutions.9, 10 Their anodic stability depends predominantly on the nature of the Al–R bonds. It was assumed that solutions comprised of complexes with phenyl groups may have positive properties in this respect. Because species such as
anions, which determine the electrochemical properties of these solutions.9, 10 Their anodic stability depends predominantly on the nature of the Al–R bonds. It was assumed that solutions comprised of complexes with phenyl groups may have positive properties in this respect. Because species such as  are not commercially available, we prepared all phenyl complex (APC) solutions by reacting PhMgCl as the Lewis base with
are not commercially available, we prepared all phenyl complex (APC) solutions by reacting PhMgCl as the Lewis base with  Lewis acid and indeed obtained solutions with apparently better performance compared to electrolyte solutions based on complexes with alkyl groups (e.g., butyl, ethyl). Our reference solution for all the measurements described herein is THF solution with
Lewis acid and indeed obtained solutions with apparently better performance compared to electrolyte solutions based on complexes with alkyl groups (e.g., butyl, ethyl). Our reference solution for all the measurements described herein is THF solution with 
 [denoted as butyl ethyl complex (BEC)]. The latter is, in fact, a formal stochiometric formula of the complex formed by a reaction between
[denoted as butyl ethyl complex (BEC)]. The latter is, in fact, a formal stochiometric formula of the complex formed by a reaction between  and
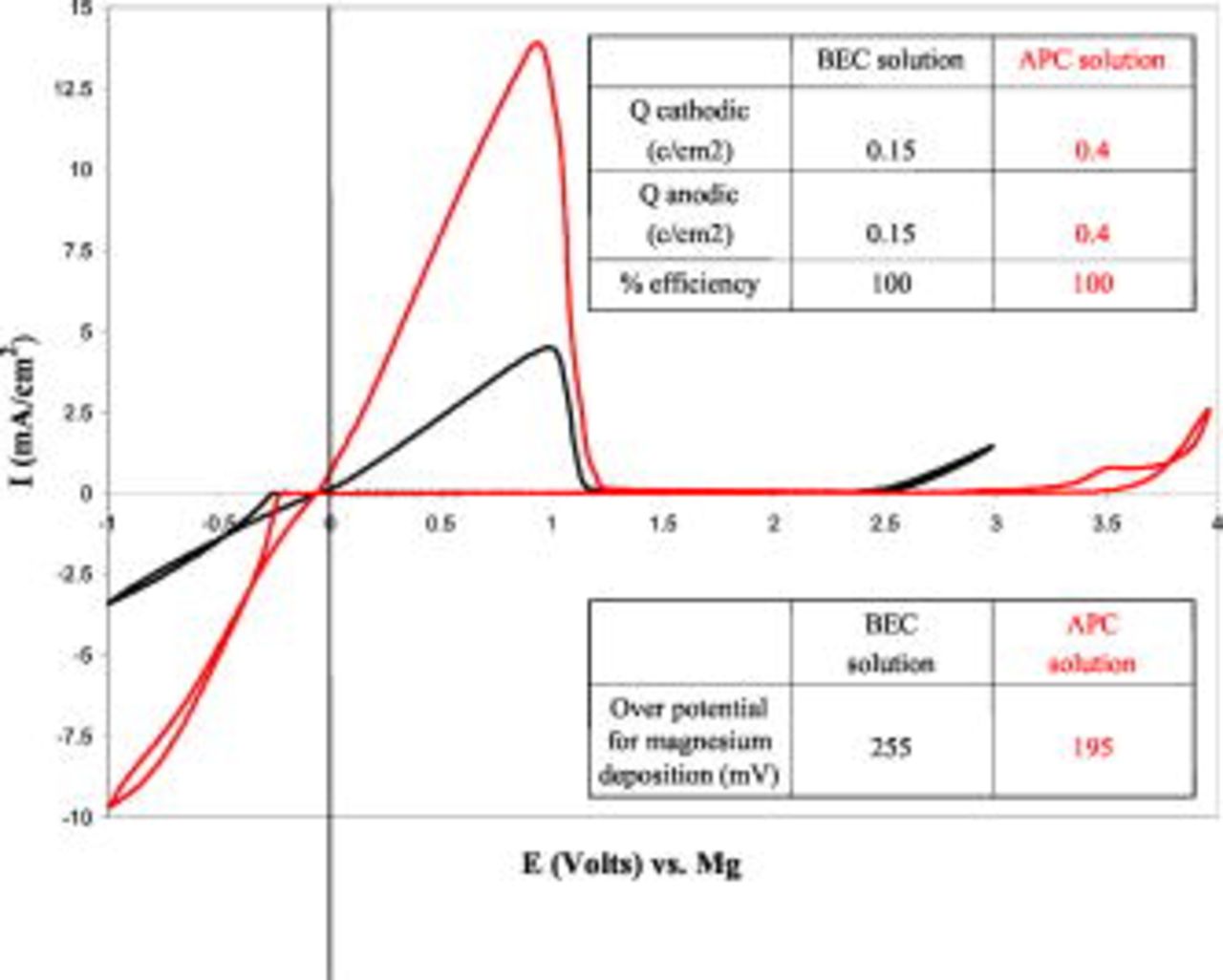
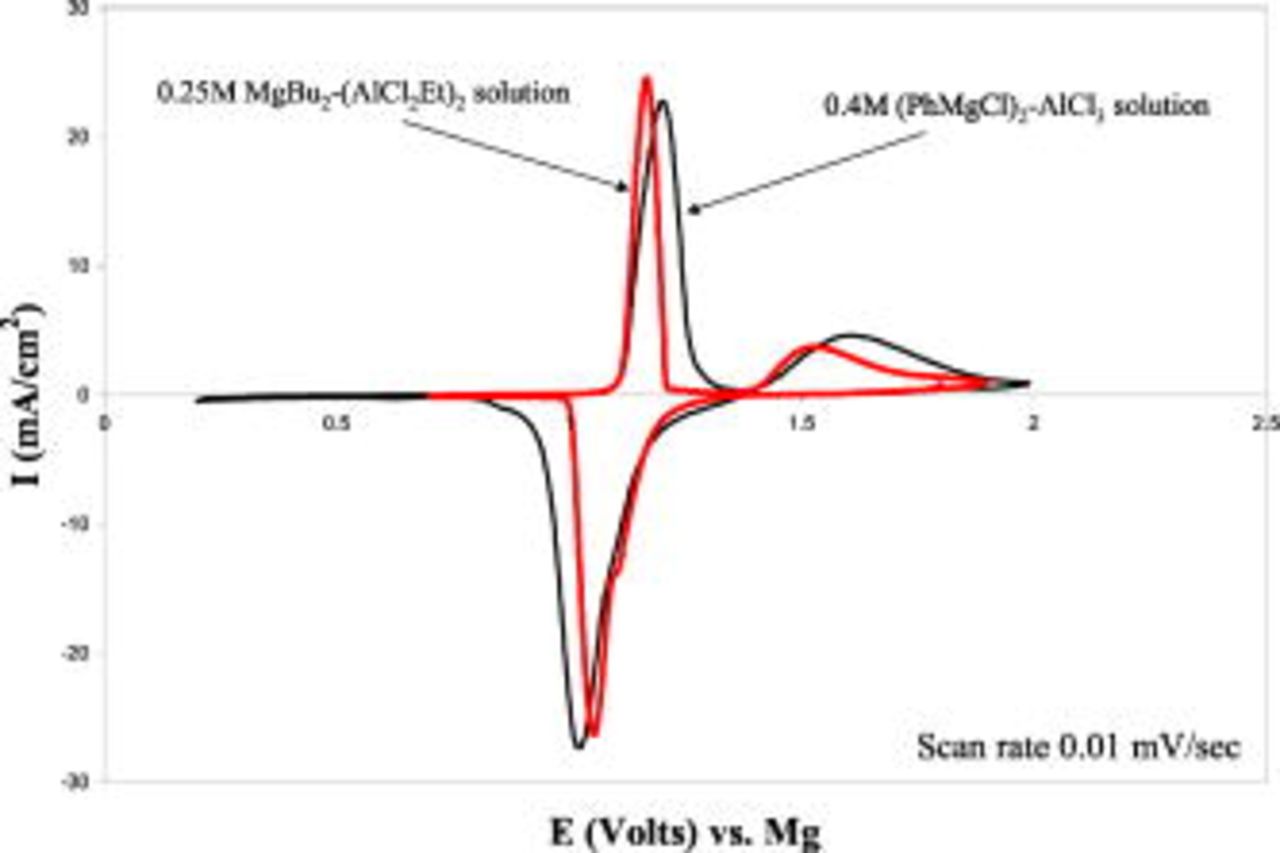
and  at a ratio of 1:2 (see description and discussion in Ref. 9, 10). This solution was the favorite electrolyte solution for the first generation of rechargeable Mg battery prototypes that was presented earlier.8, 13 Figure 1 shows a comparison between typical steady-state cyclic voltammograms obtained with
at a ratio of 1:2 (see description and discussion in Ref. 9, 10). This solution was the favorite electrolyte solution for the first generation of rechargeable Mg battery prototypes that was presented earlier.8, 13 Figure 1 shows a comparison between typical steady-state cyclic voltammograms obtained with 
 (BEC) and
(BEC) and 
 2:1 (denoted as APC) solutions in THF with Pt electrodes. The overpotentials for Mg deposition, the relevant charges of the processes, and the efficiency of Mg deposition-dissolutions are also indicated in Fig. 1. Figure 1 demonstrates the superiority of the APC over the BEC solutions in three aspects: higher anodic stability by nearly
2:1 (denoted as APC) solutions in THF with Pt electrodes. The overpotentials for Mg deposition, the relevant charges of the processes, and the efficiency of Mg deposition-dissolutions are also indicated in Fig. 1. Figure 1 demonstrates the superiority of the APC over the BEC solutions in three aspects: higher anodic stability by nearly  , lower overpotential for Mg deposition, and higher capacity for the reversible Mg deposition-dissolution processes (note that the experimental were identical in temperature, electrode's area, and potential scanning rate). The anodic stability of these solutions is determined by the Al–C bonds in the complexes, as discussed earlier.9, 10 Hence, we attribute the higher anodic stability of the all phenyl complexes in these solutions to the stronger bond between aluminum and carbon when the latter atom is part of an aromatic system.
, lower overpotential for Mg deposition, and higher capacity for the reversible Mg deposition-dissolution processes (note that the experimental were identical in temperature, electrode's area, and potential scanning rate). The anodic stability of these solutions is determined by the Al–C bonds in the complexes, as discussed earlier.9, 10 Hence, we attribute the higher anodic stability of the all phenyl complexes in these solutions to the stronger bond between aluminum and carbon when the latter atom is part of an aromatic system.
Figure 1. (Color online) Steady-state cyclic voltammograms of  BEC and
BEC and  APC solutions, measured with Pt electrodes at
APC solutions, measured with Pt electrodes at  . The charge involved, Mg cycling efficiency, and overpotential for Mg deposition are given as well.
. The charge involved, Mg cycling efficiency, and overpotential for Mg deposition are given as well.
Table I summarizes values of the overpotential for magnesium deposition and the reversibility of magnesium deposition for APC solutions (THF) of different composition and concentration measured with Pt electrodes. Similar data for a BEC, reference solution ( , THF) is also presented.
, THF) is also presented.
Table I. Overvoltage and cycling efficiency of Mg deposition, measured in different solutions as indicated. Cyclic voltammetric experiments with Pt working electrodes at  .
.
| Solution | Concentration (M) | Overpotential for magnesium deposition (mV) | Reversibility of magnesium deposition (%) |
|---|---|---|---|
1:2 MgBu2–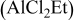 /THF /THF | 0.25 | 255 | 100 (stable) |
2:1 PhMgCl–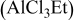 /THF /THF | 0.1 | 270 |
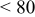
|
2:1 PhMgCl–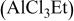 /THF /THF | 0.25 | 215 |
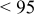
|
2:1 PhMgCl–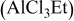 /THF /THF | 0.4 | 195 | 100 (stable) |
2:1 PhMgCl–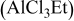 /THF /THF | 0.6 | 195 | 98 (stable) |
4:1 PhMgCl–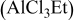 /THF /THF | 0.1 | 195 |
|
2:1 PhMgCl–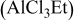 /THF /THF | 0.2 | 250 |
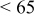
|
4:3 PhMgCl–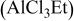 /THF /THF | 0.3 | 295 | 95 (stable) |
1:1 PhMgCl–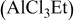 /THF /THF | 0.4 | 320 |
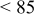
|
3:2 PhMgCl–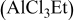 /THF /THF | 0.4 | 305 |
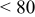
|
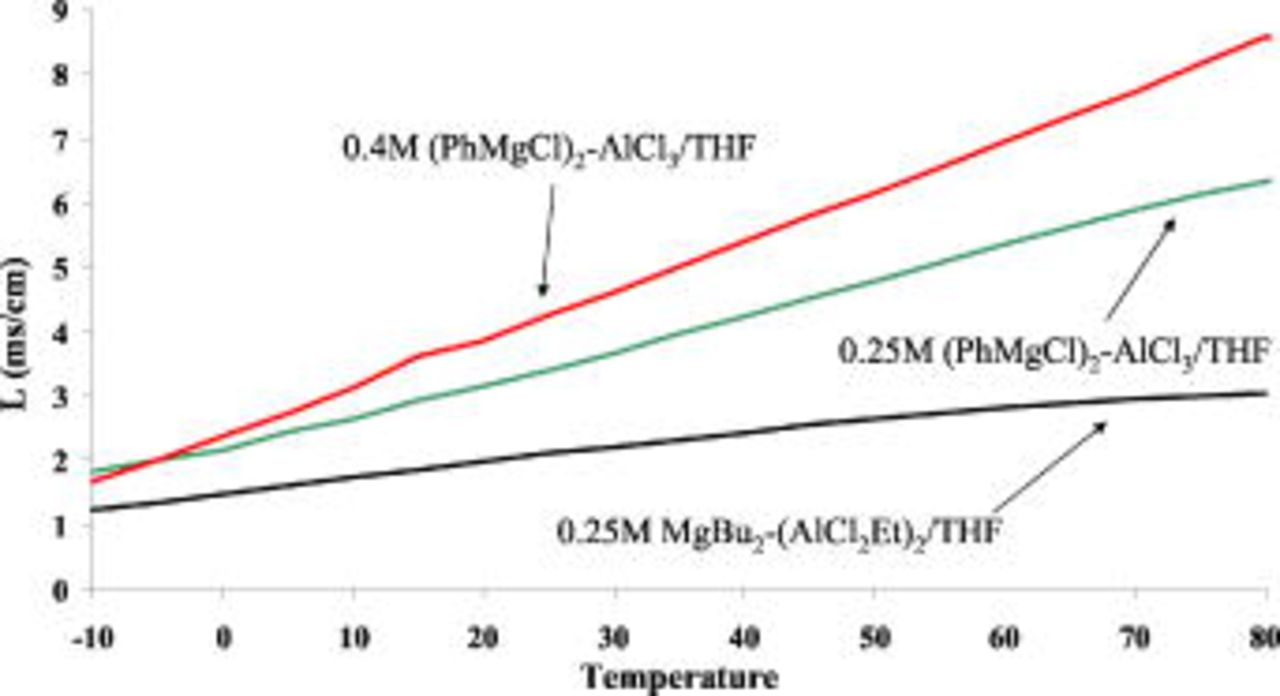
Another critical parameter of electrolyte solutions is their conductivity. Figure 2 shows the temperature dependence of the conductivity of three representative solutions (THF): 
 (BEC, the reference solution),
(BEC, the reference solution),  and
and 
 (APC), as marked on Fig. 2. The presentation in Fig. 2 speaks for itself. The latter solution demonstrates the higher specific conductivity. At room temperature
(APC), as marked on Fig. 2. The presentation in Fig. 2 speaks for itself. The latter solution demonstrates the higher specific conductivity. At room temperature  were measured, values similar to that of standard electrolyte solutions for Li batteries.14 The voltammetric experiments (Fig. 1, Table I) and the conductivity measurements (Fig. 2) show that the solutions comprising the
were measured, values similar to that of standard electrolyte solutions for Li batteries.14 The voltammetric experiments (Fig. 1, Table I) and the conductivity measurements (Fig. 2) show that the solutions comprising the  complex at concentration of
complex at concentration of  demonstrate the best properties: the lowest overpotential for Mg deposition; high anodic stability, a potential window
demonstrate the best properties: the lowest overpotential for Mg deposition; high anodic stability, a potential window  ; 100% efficiency of Mg deposition-dissolution cycling (i.e., full reversibility); and the highest conductivity. Therefore, all further electrochemical measurements were conducted using these solutions.
; 100% efficiency of Mg deposition-dissolution cycling (i.e., full reversibility); and the highest conductivity. Therefore, all further electrochemical measurements were conducted using these solutions.
Figure 2. (Color online) The temperature dependence of the specific conductivity of 
 (BEC, the reference solution), 0.25 and
(BEC, the reference solution), 0.25 and 
 (APC) solutions in THF.
(APC) solutions in THF.
Structure of electrolyte solutions
Deciphering the chemical structure of the APC solutions is important for understanding their properties. Based on previous studies of complex solutions with all alkyl ligands, it is clear that the best tool for studying the structure of these solutions is multinuclear NMR spectroscopy.11 The analysis of these solutions may suffer from the fact that there is no library of reference spectra available for possible components of these systems. Moreover, most of the relevant solution components are not readily available and have to be prepared adhoc by reacting the precursors at proportions that ensure the formation of definite dominant species (which NMR spectra can be measured and analyzed unambiguously).
Starting with  NMR, a wide peak at
NMR, a wide peak at  was observed when measuring the resonance frequency of Mg of all solutions containing the precursor PhMgCl. Therefore, it is assumed that all the solutions contain Mg ions with a coordination number of 6. It is known from previous studies that this structure might correspond to
was observed when measuring the resonance frequency of Mg of all solutions containing the precursor PhMgCl. Therefore, it is assumed that all the solutions contain Mg ions with a coordination number of 6. It is known from previous studies that this structure might correspond to  ,
,  , or
, or  .11 No indication of a Mg–C bond was observed in the
.11 No indication of a Mg–C bond was observed in the  NMR spectra of solutions containing [
NMR spectra of solutions containing [ ] complexes, which confirms that a complete transmetallation of the ligands occurs; thus, no residue of the Gringard reagent (PhMgCl) remains in solutions.
] complexes, which confirms that a complete transmetallation of the ligands occurs; thus, no residue of the Gringard reagent (PhMgCl) remains in solutions.
Specific structural information was obtained from  and
and  NMR measurements, with the latter being the most useful. The
NMR measurements, with the latter being the most useful. The  NMR spectra of these solutions are usually characterized by broad peaks. However, when the symmetry of the molecules is tetrahedral, a narrowing of the peak width is seen. Therefore, by examining the peak width, one can determine the symmetry of the Al atoms in the ions/molecules in solutions. Apart from the coordination number and the symmetry of Al atoms in the ions/molecules, the chemical shifts might point to the nature of Al ligands, namely, as the number of the inorganic ligands (Cl) is higher, the chemical shift should be lower (hence, as the number of the organic ligands is higher, the chemical shift should be higher).11
NMR spectra of these solutions are usually characterized by broad peaks. However, when the symmetry of the molecules is tetrahedral, a narrowing of the peak width is seen. Therefore, by examining the peak width, one can determine the symmetry of the Al atoms in the ions/molecules in solutions. Apart from the coordination number and the symmetry of Al atoms in the ions/molecules, the chemical shifts might point to the nature of Al ligands, namely, as the number of the inorganic ligands (Cl) is higher, the chemical shift should be lower (hence, as the number of the organic ligands is higher, the chemical shift should be higher).11
In order to assign each  peak to the corresponding species, a series of solutions of different compositions and concentrations was analyzed. The NMR spectra of
peak to the corresponding species, a series of solutions of different compositions and concentrations was analyzed. The NMR spectra of  (the Lewis acid precursor of the APC solutions) in THF, show a single peak at a chemical shift of
(the Lewis acid precursor of the APC solutions) in THF, show a single peak at a chemical shift of  for unsaturated solutions. With a saturated
for unsaturated solutions. With a saturated  solution, an additional peak is observed at
solution, an additional peak is observed at  , corresponding to a
, corresponding to a  dimer.
dimer.
We assume that reacting PhMgCl and  at high base to acid ratio should form
at high base to acid ratio should form  as the main anionic species. The
as the main anionic species. The  NMR spectrum of
NMR spectrum of  solutions, show a single peak at
solutions, show a single peak at  . This peak should be attributed
. This peak should be attributed  , which is the expected product of transmetallation of the ligands between Mg and Al, at a high ratio between the Gignard reagent (PhMgCl) and the
, which is the expected product of transmetallation of the ligands between Mg and Al, at a high ratio between the Gignard reagent (PhMgCl) and the  Lewis acid. The following reaction scheme is suggested to such solutions
Lewis acid. The following reaction scheme is suggested to such solutions
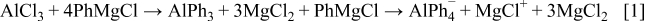
Reacting PhMgCl and  at high acid/base ratio, we expect the formation of species such as
at high acid/base ratio, we expect the formation of species such as  and
and  , according to the following scheme
, according to the following scheme
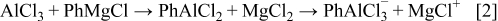
The  spectra of
spectra of  solutions (e.g.,
solutions (e.g.,  ) show the expected
) show the expected  peak at
peak at  and a second broad peak at
and a second broad peak at  . It is most logical to attribute this peak with the medium shift (between that of
. It is most logical to attribute this peak with the medium shift (between that of  and
and  ) to
) to  and/or
and/or  .
.
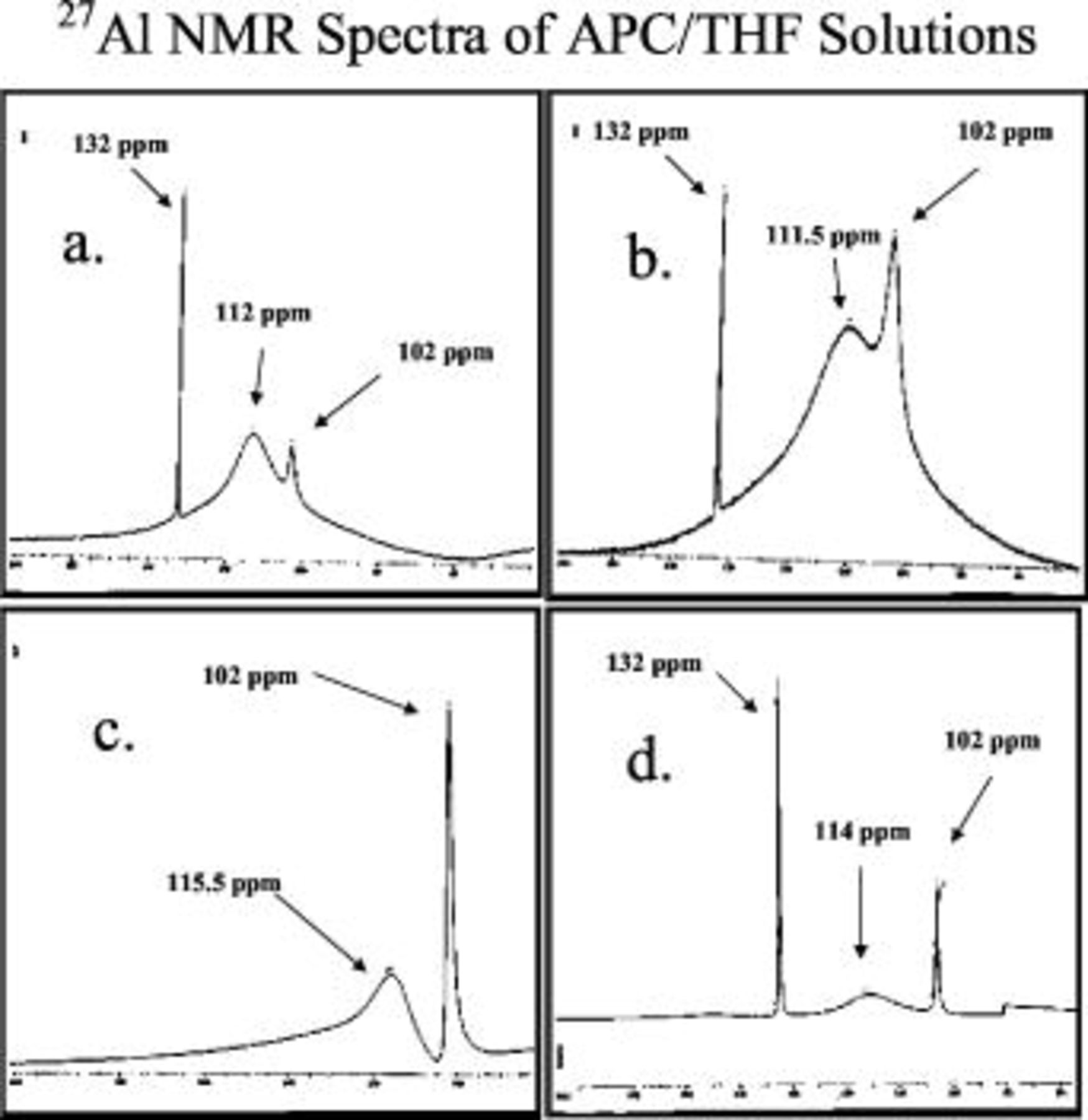
In the next step, solutions with compositions of  4:3, 1:1, 3:2, and 2:1 (THF) were analyzed. Figure 3 shows the
4:3, 1:1, 3:2, and 2:1 (THF) were analyzed. Figure 3 shows the  NMR spectra of these
NMR spectra of these  solutions with a different ratio between the reagents. The peaks corresponding to the
solutions with a different ratio between the reagents. The peaks corresponding to the  dimer
dimer  and to the
and to the  species
species  were observed plus peaks around
were observed plus peaks around  that belong to aluminum, which is bound to both chloride and phenyl ligands, where the higher chemical
that belong to aluminum, which is bound to both chloride and phenyl ligands, where the higher chemical  higher number of phenyl ligands in the Al species. The selection of the precursors ratios in the solutions measured indeed enabled differentiation among the various species formed in each solution. Table II presents the species assigned to the various
higher number of phenyl ligands in the Al species. The selection of the precursors ratios in the solutions measured indeed enabled differentiation among the various species formed in each solution. Table II presents the species assigned to the various  peaks in the spectra of Fig. 3. In addition,
peaks in the spectra of Fig. 3. In addition,  NMR spectra of these solutions were measured. They include peaks in the domains
NMR spectra of these solutions were measured. They include peaks in the domains  (ring carbons close to Al) and
(ring carbons close to Al) and  (ring carbons far from Al). They are much less conclusive, but generally in line with the
(ring carbons far from Al). They are much less conclusive, but generally in line with the  spectra. Based on the spectra of the above four solutions (see Fig. 3), it was possible to analyze, quite precisely, the reactions and equilibria that take place in each solution. Consequently, Scheme 1 provides the relevant reaction equations for each of the four solutions.
spectra. Based on the spectra of the above four solutions (see Fig. 3), it was possible to analyze, quite precisely, the reactions and equilibria that take place in each solution. Consequently, Scheme 1 provides the relevant reaction equations for each of the four solutions.
Figure 3.
 NMR spectra measured with APC solution of the following compositions
NMR spectra measured with APC solution of the following compositions  : (a) 4:3, (b) 1:1, (c) 3:2, and (d) 2:1.
: (a) 4:3, (b) 1:1, (c) 3:2, and (d) 2:1.
Table II. Shift and width of  NMR peaks of various aluminum compounds that are formed in APC solutions, based on the measurements of solutions with different ratios between PhMgCl and
NMR peaks of various aluminum compounds that are formed in APC solutions, based on the measurements of solutions with different ratios between PhMgCl and  .
.
| Species | Peaks shift (ppm) | Peaks width (Hz) |
|---|---|---|
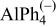
| 132 | 25 |
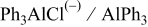
| 115 | 125 |
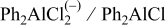
| 112 | 2000 |
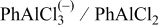
| 91 | 1460 |
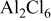
| 102 | 500 |
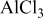
| 62 | 1500 |
Scheme 1
Reaction paths and final composition of several  solutions with different reagents ratios as indicated, based on their NMR spectra (Fig. 3).
solutions with different reagents ratios as indicated, based on their NMR spectra (Fig. 3).
- (a)Solutions comprising PhMgCl and
 in a ratio of 4:3
in a ratio of 4:3 - (b)Solutions comprising PhMgCl and
 in a ratio of 1:1
in a ratio of 1:1 - (c)Solutions comprising PhMgCl and
 in a ratio of 3:2
in a ratio of 3:2 - (d)Solution comprising PhMgCl and
 in a ratio of 2:1 (that of the best performance)
in a ratio of 2:1 (that of the best performance)
In Eq. 1, 2 and in Scheme 1, the species  and
and  appear as both products and intermediates. It should be noted that, based on the previous work,8–12 it is clear that the cationic species in these solutions have complex structures.
appear as both products and intermediates. It should be noted that, based on the previous work,8–12 it is clear that the cationic species in these solutions have complex structures.  and
and  form together the cation
form together the cation  . All the above Mg–Cl moieties are stabilized by the ether molecules that also play an important role in the structures of both the cations and the anions in these solutions. Hence, the reactions paths and products distribution in Eq. 1, 2 and Scheme 1 provide a first approximation for the map of reactions possible in these solutions. The real picture may be more complicated because more equilibrium reactions (beyond what is suggested above) may be relevant to each of the solutions discussed above. In any event, as can be seen from Scheme 1, the solutions of the best performance, prepared with the precursors PhMgCl and
. All the above Mg–Cl moieties are stabilized by the ether molecules that also play an important role in the structures of both the cations and the anions in these solutions. Hence, the reactions paths and products distribution in Eq. 1, 2 and Scheme 1 provide a first approximation for the map of reactions possible in these solutions. The real picture may be more complicated because more equilibrium reactions (beyond what is suggested above) may be relevant to each of the solutions discussed above. In any event, as can be seen from Scheme 1, the solutions of the best performance, prepared with the precursors PhMgCl and  at a ratio of 2:1, comprise obviously
at a ratio of 2:1, comprise obviously  ,
,  , and
, and  and/or
and/or  as main active species. The lack of species with Mg⎯C bonds, ensures a reasonable anodic stability for these systems, which is higher by
as main active species. The lack of species with Mg⎯C bonds, ensures a reasonable anodic stability for these systems, which is higher by  compared to solutions based on complexes with alkyl ligands.8–12 Hence, one can conclude that organoaluminate species in which the ligands are aromatic, develop stronger Al⎯C bonds, compared to aliphatic systems. One can also conclude that the
compared to solutions based on complexes with alkyl ligands.8–12 Hence, one can conclude that organoaluminate species in which the ligands are aromatic, develop stronger Al⎯C bonds, compared to aliphatic systems. One can also conclude that the 
 anions thus formed are indeed more stable than the
anions thus formed are indeed more stable than the  anions,
anions,  , which are the active anionic species in the solutions that were explored and tested previously.8–12 Their relatively high stability ensures a very good ion separation in solutions, which explains well both the relatively high specific conductivity and the improved parameters of Mg deposition (relatively low overpotentials and cycling efficiency of 100%).
, which are the active anionic species in the solutions that were explored and tested previously.8–12 Their relatively high stability ensures a very good ion separation in solutions, which explains well both the relatively high specific conductivity and the improved parameters of Mg deposition (relatively low overpotentials and cycling efficiency of 100%).
Morphology of Mg deposition
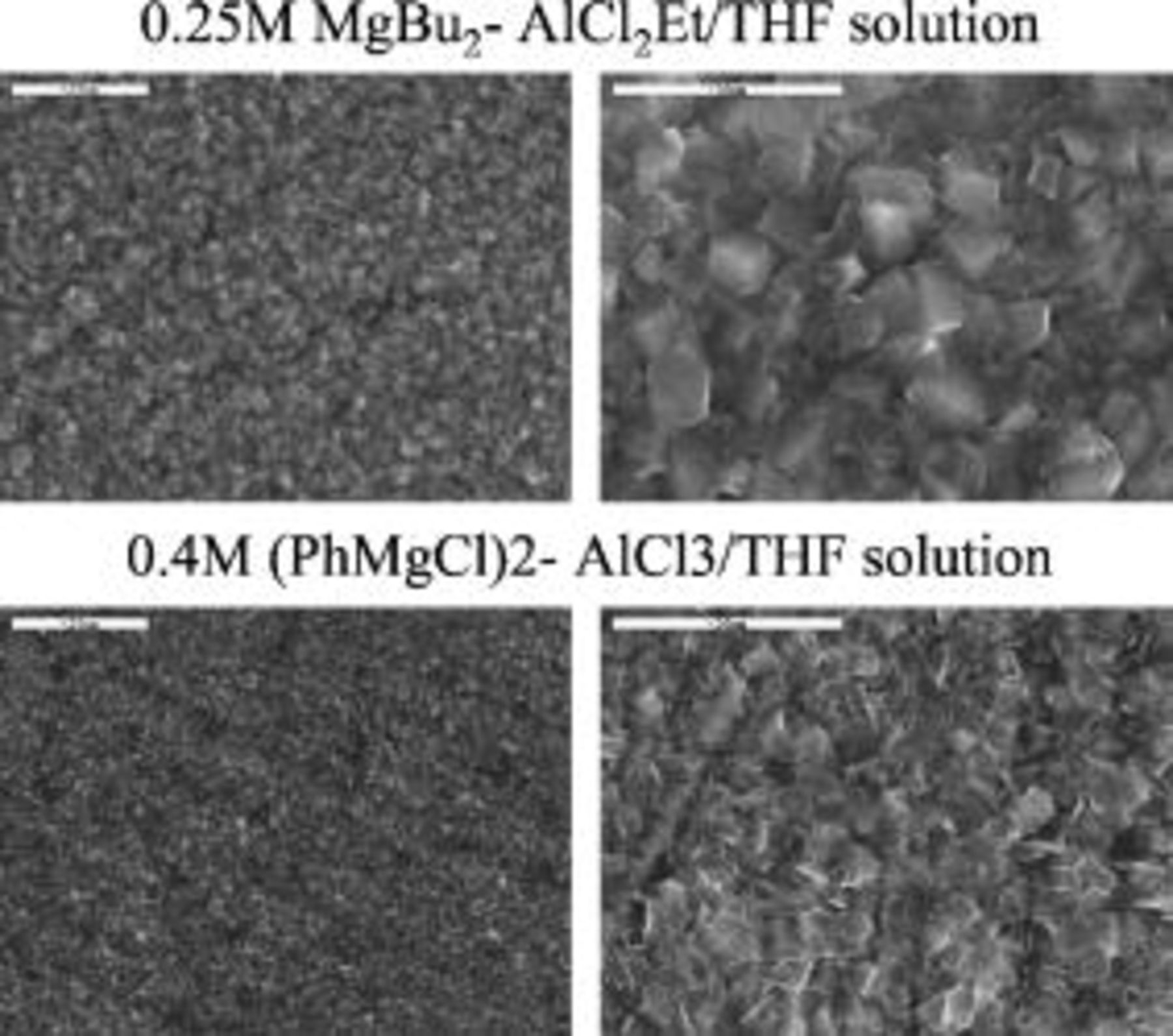
Figure 4 shows two pairs of SEM images at two magnifications (indicated) of magnesium deposits on Cu substrates from 
 (BEC) and
(BEC) and  [
[ 9APC] solutions, the former solution showing the best performance in our previous studies8–12 and the latter one being the favorite electrolyte solution in the present study. The deposition rates were at
9APC] solutions, the former solution showing the best performance in our previous studies8–12 and the latter one being the favorite electrolyte solution in the present study. The deposition rates were at  with a total charge of a few coulmbs per square centimeter. EDS measurements of the deposits confirmed that they are pure magnesium. These morphological measurements by SEM showed that Mg deposition from both solutions is very uniform. However, in general, the size of the Mg particles deposited from the APC solutions was found to be smaller (submicronic in average) compared to the Mg deposits from the BEC solutions (usually
with a total charge of a few coulmbs per square centimeter. EDS measurements of the deposits confirmed that they are pure magnesium. These morphological measurements by SEM showed that Mg deposition from both solutions is very uniform. However, in general, the size of the Mg particles deposited from the APC solutions was found to be smaller (submicronic in average) compared to the Mg deposits from the BEC solutions (usually  , on average). This uniform Mg deposition morphology is very important for practical use because it ensures the lack of dendrite formation in battery systems (provided that their engineering aspects are optimal).
, on average). This uniform Mg deposition morphology is very important for practical use because it ensures the lack of dendrite formation in battery systems (provided that their engineering aspects are optimal).
Figure 4. SEM images of Mg deposits on copper substrates in BEC  and APC (0.4) solutions as indicated. Two magnifications, a scale appears in each image. Current density
and APC (0.4) solutions as indicated. Two magnifications, a scale appears in each image. Current density  , total charge
, total charge  .
.
Anodic stability, the performance of Mg insertion cathodes and Mg battery prototypes
APC solutions were electrolyzed during several hours at potentials  ,
,  beyond their anodic limit, and the solutions after prolonged electrolysis were analyzed by NMR spectroscopy. A highly important result is the lack of any gas formation during these experiments, which is an important finding in terms of the safety features of these solutions. The main products of this electrolysis that could be easily detected by NMR are benzene and biphenyl. Solvent molecules are probably also involved in the oxidation of these solution, however, a rigorous study of these oxidation reactions is beyond the scope of this work.
beyond their anodic limit, and the solutions after prolonged electrolysis were analyzed by NMR spectroscopy. A highly important result is the lack of any gas formation during these experiments, which is an important finding in terms of the safety features of these solutions. The main products of this electrolysis that could be easily detected by NMR are benzene and biphenyl. Solvent molecules are probably also involved in the oxidation of these solution, however, a rigorous study of these oxidation reactions is beyond the scope of this work.
The next step was to study the Mg ions intercalation from the new APC solutions  electrodes, which are suggested as possible cathodes for rechargeable Mg batteries. Figure 5 exhibits steady state slow scanning rate cyclic voltammograms of composite
electrodes, which are suggested as possible cathodes for rechargeable Mg batteries. Figure 5 exhibits steady state slow scanning rate cyclic voltammograms of composite  electrodes (comprising 80% active mass, 10% PVDF binder, and 10% carbon black)15 in BEC and APC solutions as indicated (vs Mg foils reference and counter electrodes). In general, the voltammetric study of these systems showed that Mg ions intercalate reversibly into
electrodes (comprising 80% active mass, 10% PVDF binder, and 10% carbon black)15 in BEC and APC solutions as indicated (vs Mg foils reference and counter electrodes). In general, the voltammetric study of these systems showed that Mg ions intercalate reversibly into 
 electrodes in two stages8, 15 (the voltammograms include two sets of peaks where the two cathodic peaks overlap). As expected, the practical capacity of these cathodes in both solutions is lower at room temperature by 20–25% from the theoretical value
electrodes in two stages8, 15 (the voltammograms include two sets of peaks where the two cathodic peaks overlap). As expected, the practical capacity of these cathodes in both solutions is lower at room temperature by 20–25% from the theoretical value  due to the well-discussed and understood partial charge trapping phenomenon.16, 17 The peaks' separation in the voltammogram related to the APC solution is wider than that observed for the BEC solution (Fig. 5), which indicates a more sluggish kinetics of Mg ions insertion into these cathodes in the former solutions (which reason was not yet explored).
due to the well-discussed and understood partial charge trapping phenomenon.16, 17 The peaks' separation in the voltammogram related to the APC solution is wider than that observed for the BEC solution (Fig. 5), which indicates a more sluggish kinetics of Mg ions insertion into these cathodes in the former solutions (which reason was not yet explored).
Figure 5. (Color online) Comparison of typical steady-state, slow scan rate cyclic voltammograms measured with composite  electrodes (80% active mass, 10% PVDF binder, 10% carbon black) in standard BEC
electrodes (80% active mass, 10% PVDF binder, 10% carbon black) in standard BEC  and APC
and APC  solutions as indicated.
solutions as indicated.  .
.
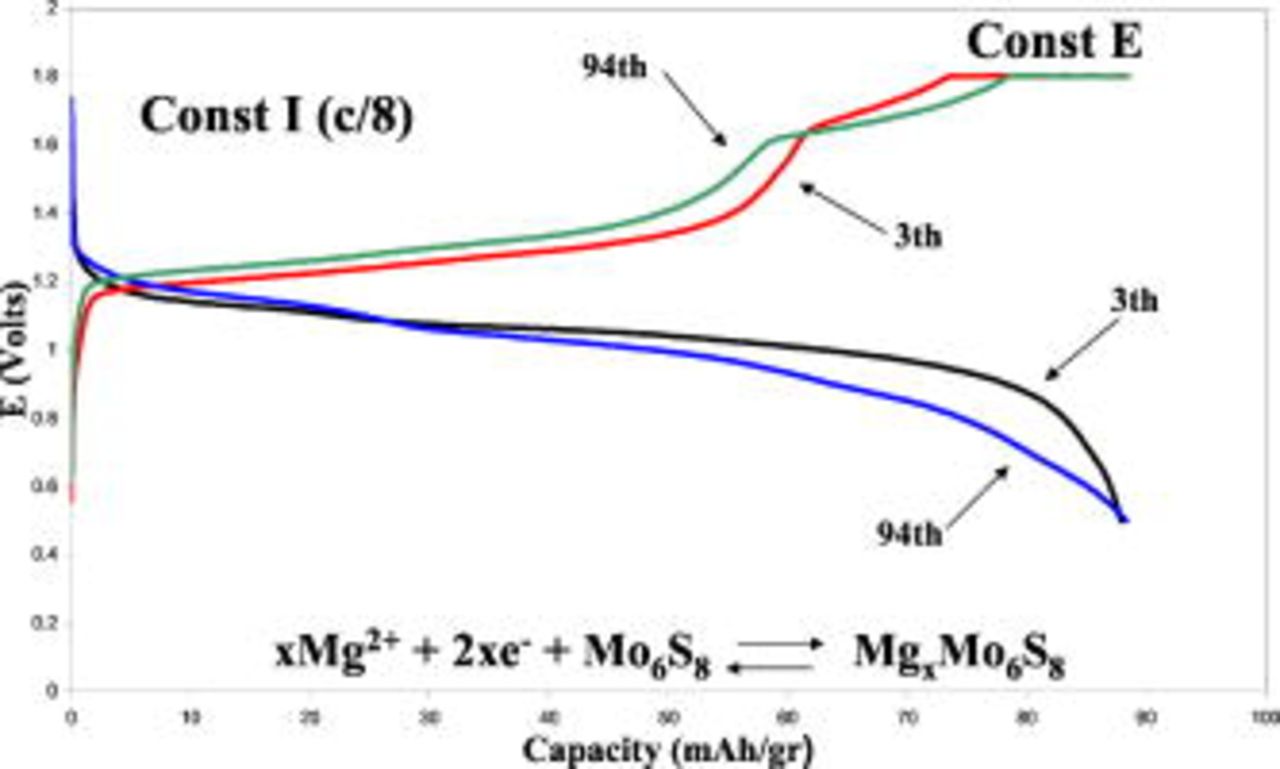
Preliminary testing of Mg battery prototypes, comprising Mg foil anodes, composite Mo6S8 Chevrel phase cathodes, and APC solution, in coin type cells, was carried out. Figure 6 compares typical voltage profiles of these cells at the 3rd and 94th cycles. Although there are slight changes in the shape of the voltage profiles as cycling proceeds, the capacity, calculated based on the weight of the cathode's active mass, remains stable. It should be noted that the data in Fig. 6 relates to unoptimized cells, with micrometric size particles. As demonstrated in a parallel publication,18 it is possible to synthesize improved Chevrel phase cathode materials, with which the partial charge trapping phenomenon is alleviated and capacities close to the theoretical value can be obtained. In general, these prototype batteries could be cycled hundreds of times showing stable performance. In these battery systems, there are no side reactions. Mg electrodes are completely inert and passivation-free in all of these complex electrolyte-ethereal solutions. Also, the redox activity of the Chevrel phase cathodes falls well within the electrochemical window of these solutions with no side reactions as well. Thereby, it is expected that these systems should have very prolonged cyclability, better than all other rechargeable battery systems known to date. However, the duration of the experiments carried out in the framework of the present study was limited due to inevitable evaporation of the THF solvent through the plastic gaskets of the coin-type cells.
Figure 6. (Color online) Typical voltage profiles of rechargeable Mg batteries in coin-type cells. Mg foil anode, composite  cathode (80% active mass, 10% PVDF binder, 10% carbon black), and optimal APC solution [
cathode (80% active mass, 10% PVDF binder, 10% carbon black), and optimal APC solution [
 ]. The data of the 3rd and the 94th cycles are presented. The capacity is calculated per the cathode's active mass. Discharge rate: C/8 rate (galvanostatic). Charging: galvanostatic at C/8, followed by
]. The data of the 3rd and the 94th cycles are presented. The capacity is calculated per the cathode's active mass. Discharge rate: C/8 rate (galvanostatic). Charging: galvanostatic at C/8, followed by  at constant voltage as indicated.
at constant voltage as indicated.
Thermal studies of electrolyte solutions and Mg batteries electrodes by DSC
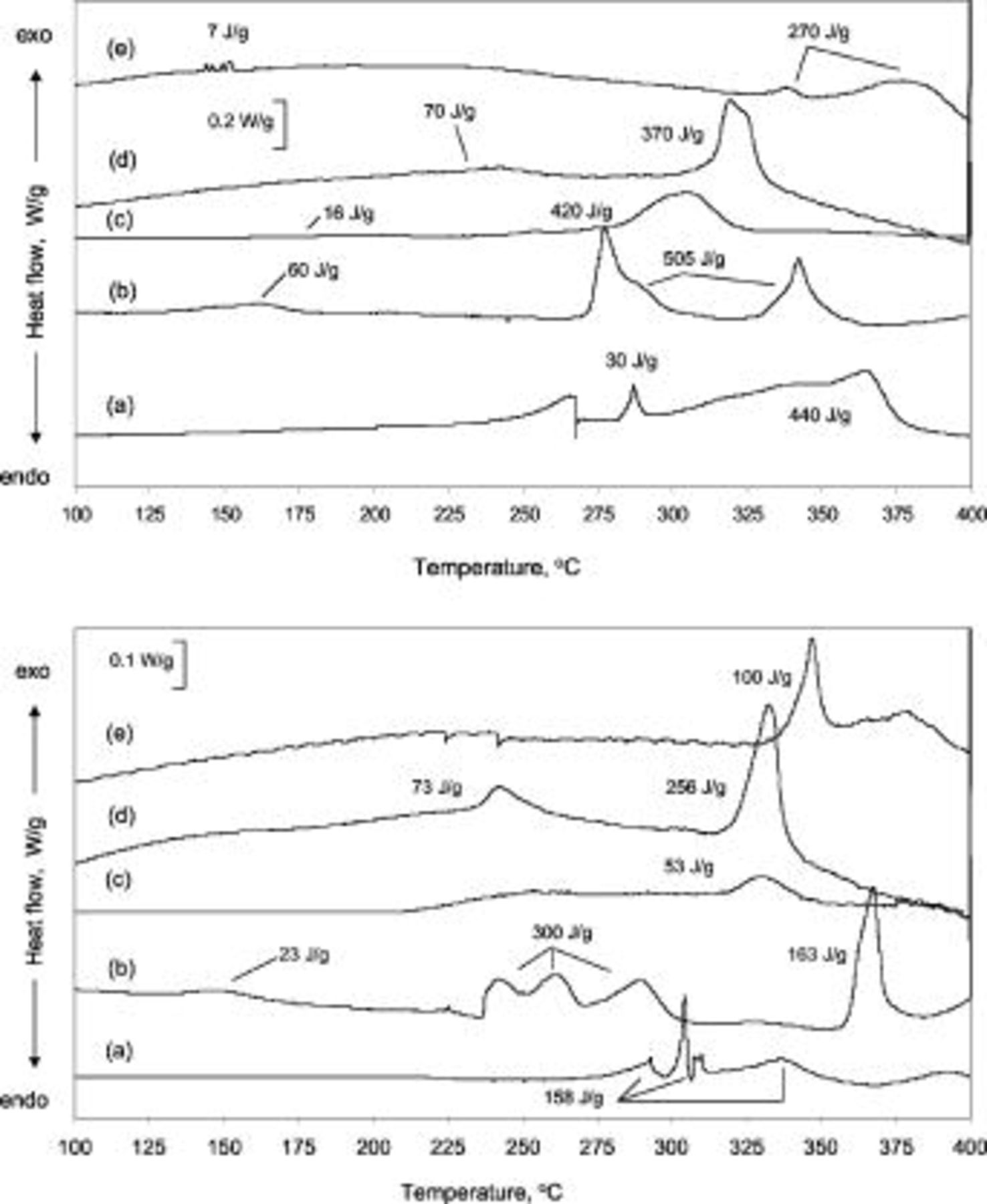
Figures 7a and 7b compare the thermal behavior of APC and BEC solutions, respectively, studied by DSC. As presented in Fig. 7, five sets of experiments were carried out: the solutions themselves,  foil,
foil,  ,
,  and all batteries
and all batteries  . Heat flow (watts per gram solution) for all the thermal processes in the above systems is plotted as a function of temperature (in the course of heating at a constant rate of
. Heat flow (watts per gram solution) for all the thermal processes in the above systems is plotted as a function of temperature (in the course of heating at a constant rate of  ). Each of the 10 curves presented in Fig. 7a and 7b exhibits several exothermal processes appearing as positive peaks. The relevant specific heats involved in the main processes are indicated near the peaks. In general, the thermal behavior of the two systems based on BEC and APC solutions is somewhat similar. The pure solutions show some thermal activity at temperatures above
). Each of the 10 curves presented in Fig. 7a and 7b exhibits several exothermal processes appearing as positive peaks. The relevant specific heats involved in the main processes are indicated near the peaks. In general, the thermal behavior of the two systems based on BEC and APC solutions is somewhat similar. The pure solutions show some thermal activity at temperatures above  . The APC solutions exhibit an interesting start around
. The APC solutions exhibit an interesting start around  : an exotherm start and continues as an endothermic process as heating proceeds (see the positive shoulder in the first curve of Fig. 7a, which is followed by a spike that goes down). The main thermal processes of both solutions are exothermic reactions that emit a few hundreds of Joules per gram take place (have not yet been explored). These solutions demonstrate much higher thermal stability than standard solutions for Li ion batteries, which exhibit pronounced exothermic redox activity with onset temperatures below
: an exotherm start and continues as an endothermic process as heating proceeds (see the positive shoulder in the first curve of Fig. 7a, which is followed by a spike that goes down). The main thermal processes of both solutions are exothermic reactions that emit a few hundreds of Joules per gram take place (have not yet been explored). These solutions demonstrate much higher thermal stability than standard solutions for Li ion batteries, which exhibit pronounced exothermic redox activity with onset temperatures below  and with specific heat emission several times higher than that of the BEC or APC ethereal solutions.19 As expected, mixtures of the above solutions with Mg metal, exhibit more intensive thermal activity than the neat solutions (see the second curves in Fig. 7a and 7b). This includes a minor process around
and with specific heat emission several times higher than that of the BEC or APC ethereal solutions.19 As expected, mixtures of the above solutions with Mg metal, exhibit more intensive thermal activity than the neat solutions (see the second curves in Fig. 7a and 7b). This includes a minor process around  , and two sets of main thermal processes are measured in the temperature ranges 225–300 and
, and two sets of main thermal processes are measured in the temperature ranges 225–300 and  for both systems. The onset temperature of the main thermal process of the APC/Mg system is higher by
for both systems. The onset temperature of the main thermal process of the APC/Mg system is higher by  than that for the BEC/Mg system. This thermal behavior of the THF/APC or THF/BEC with magnesium is much weaker in terms of higher onset and lower heat generation, compared to mixtures of standard solutions for Li or Li ion batteries with anode materials such as Li or lithiated graphite.20, 21 The thermal behavior of APC solutions with
than that for the BEC/Mg system. This thermal behavior of the THF/APC or THF/BEC with magnesium is much weaker in terms of higher onset and lower heat generation, compared to mixtures of standard solutions for Li or Li ion batteries with anode materials such as Li or lithiated graphite.20, 21 The thermal behavior of APC solutions with  or
or  powders exhibits exothermic reactions at onset temperatures higher than 275 and
powders exhibits exothermic reactions at onset temperatures higher than 275 and  , respectively.
, respectively.
Figure 7. (Color online) (top) DSC profiles (power emitted vs temperature in the course of continuous heating) and specific heat of reactions (marked near the peaks) for (a)  of APC solution, (b)
of APC solution, (b)  of APC solution in contact with
of APC solution in contact with  of Mg metal, (c)
of Mg metal, (c)  of APC solution in contact with
of APC solution in contact with 
 Chevrel phase, (d)
Chevrel phase, (d)  of APC solution with
of APC solution with 
 Chevrel phase, and (e)
Chevrel phase, and (e)  of APC solution in contact with
of APC solution in contact with  Mg metal and
Mg metal and 
 . (bottom) DSC profiles (power emitted vs temperature in the course of continuous heating) and specific heat of reactions (marked near the peaks) for (a)
. (bottom) DSC profiles (power emitted vs temperature in the course of continuous heating) and specific heat of reactions (marked near the peaks) for (a)  of BEC solution, (b)
of BEC solution, (b)  of BEC solution in contact with
of BEC solution in contact with  of Mg metal, (c)
of Mg metal, (c)  of BEC solution with
of BEC solution with 
 Chevrel phase, (d)
Chevrel phase, (d)  of BEC solution with
of BEC solution with 
 Chevrel phase, and (e)
Chevrel phase, and (e)  of BEC solution in contact with
of BEC solution in contact with  Mg metal and
Mg metal and 
 .
.
The APC solutions seem to be more stable thermally with the Chevrel phase (CP) cathode materials than mixtures based on BEC solutions. It should be noted that the mixture of APC or BEC/THF solutions with the CP cathodes are much more thermally stable than mixtures of standard solutions for Li ion batteries and Li insertion cathodes such as  .22, 23 Highly interesting is the fact that the weakest thermal activity was observed for both electrolyte solutions (APC, BEC) when the mixtures contained solutions with both Mg metal and
.22, 23 Highly interesting is the fact that the weakest thermal activity was observed for both electrolyte solutions (APC, BEC) when the mixtures contained solutions with both Mg metal and  . This finding is important because it reflects encouraging safety features for rechargeable Mg batteries, especially because the ratio among the components (Mg metal, cathode's active mass, and electrolyte solution) in these experiments was not too far from that which exists in practical battery systems. Because the possible high temperature reactions of these systems have not been explored yet (beyond the scope of this study), we cannot explain the thermal behavior of the systems, presented in Fig. 7. However, we encounter here an interesting phenomenon: due to some interrelation between their thermal reactions with the solutions, the presence of both anode and cathode components in these systems damps the overall thermal activity, compared to that of the binary (solution/Mg or solution
. This finding is important because it reflects encouraging safety features for rechargeable Mg batteries, especially because the ratio among the components (Mg metal, cathode's active mass, and electrolyte solution) in these experiments was not too far from that which exists in practical battery systems. Because the possible high temperature reactions of these systems have not been explored yet (beyond the scope of this study), we cannot explain the thermal behavior of the systems, presented in Fig. 7. However, we encounter here an interesting phenomenon: due to some interrelation between their thermal reactions with the solutions, the presence of both anode and cathode components in these systems damps the overall thermal activity, compared to that of the binary (solution/Mg or solution  ) systems.
) systems.
Conclusion
We report herein on the development of electrolyte solutions for rechargeable magnesium batteries in which Mg electrochemistry is fully reversible: Mg deposition-dissolution processes can be cycled at low overpotential and 100% cycling efficiency, with a smooth and uniform Mg deposition morphology. The conductivity of these solutions is similar to that of standard solutions for Li batteries and thus can be considered as high for nonaqueous electrolyte solutions. Especially important is their wide electrochemical window,  . These solutions are prepared from relatively cheap and commercially available precursors,
. These solutions are prepared from relatively cheap and commercially available precursors,  and
and  in THF, which undergo Lewis acid–Lewis base reactions in solutions, in which transmetallation of the ligands occurs. Hence, the active species in solutions are cations of the type
in THF, which undergo Lewis acid–Lewis base reactions in solutions, in which transmetallation of the ligands occurs. Hence, the active species in solutions are cations of the type  or
or  and anions of the type
and anions of the type  and
and 
 . Based on previous work, it is clear that THF molecules are also part of the actual structure of the active species in solutions. It can be concluded that the Al–C bond is stronger for phenyl than for alkyl ligands, which explains the improved anodic stability of these electrolyte solutions and their improved conductivity as well: if the Al–Ph-based anions are more stable, the ion separation in solutions should be better.
. Based on previous work, it is clear that THF molecules are also part of the actual structure of the active species in solutions. It can be concluded that the Al–C bond is stronger for phenyl than for alkyl ligands, which explains the improved anodic stability of these electrolyte solutions and their improved conductivity as well: if the Al–Ph-based anions are more stable, the ion separation in solutions should be better.
The behavior of Mg intercalation cathode materials, namely  (CP), in the solutions is similar to that found in the first-generation solutions, based on complexes with alkyl ligands: fully reversible Mg insertion is observed, at capacities
(CP), in the solutions is similar to that found in the first-generation solutions, based on complexes with alkyl ligands: fully reversible Mg insertion is observed, at capacities  of that of the theoretical capacity, due to a partial (constant) Mg ions trapping that occurs at low temperatures. This problem can be alleviated by the use of modified CP electrodes, as reported in parallel publications. Finally, the new solutions exhibit improved safety features in terms of well-damped thermal activity and overcharge behavior.
of that of the theoretical capacity, due to a partial (constant) Mg ions trapping that occurs at low temperatures. This problem can be alleviated by the use of modified CP electrodes, as reported in parallel publications. Finally, the new solutions exhibit improved safety features in terms of well-damped thermal activity and overcharge behavior.
The use of aromatic ligands enables one to modify the solution properties further by the use of appropriate substitution (e.g., by electron withdrawing groups that can increase even further the anodic stability). The high anodic stability of the solutions presented herein, opens the door for the use of other possible Mg insertion cathode material of higher voltage and capacity than that of Chevrel phases, which may pave the way for research and development of high-energy density, rechargeable Mg battteries.
Acknowledgments
Partial support for this work was obtained from the BSF, Israel-US Bi-national Science Foundation and from the GIF, Israel-Germany Science Foundation.
Bar-Ilan University assisted in meeting the publication costs of this article.