Abstract
A large-area network of polyaniline nanowires was potentiostatically deposited on a stainless steel electrode at the potential of  and characterized in
and characterized in 
 electrolyte for a redox supercapacitor. The specific capacitance of
electrolyte for a redox supercapacitor. The specific capacitance of  and specific power of
and specific power of  was obtained at the charge-discharge current density of
was obtained at the charge-discharge current density of  . A long charge-discharge cyclic stability of the polyaniline nanowires is observed.
. A long charge-discharge cyclic stability of the polyaniline nanowires is observed.
Export citation and abstract BibTeX RIS
In the wake of increasing pollution and depleting oil reserves, the requirement for the supercapacitors as alternative sources of high power has been gaining momentum for the last decade. The recent explosive growth in the portable electronic devices market pushed the development of supercapacitors as the highest priority for high power applications.1–5 Supercapacitors are charge-storage devices, which possess high power density, excellent reversibility, and have longer cycle-life as compared to batteries. Superpcapacitors derive their power from the charge-storage at the electrode∕electrolyte interface and can be classified as follows: (i) redox supercapacitors, which utilize the pseudocapacitance arising from reversible faradic reactions occurring at the electrode surface and (ii) electrical double-layer capacitors, which utilize the capacitance arising from charge separation at the electrode∕electrolyte interface. The key to the high specific power of a supercapacitor lies in the nature as well as the surface area of its electrode material. The ability of a supercapacitor to supply high power lies in the charge-storage occurring in the nanosize thick region at the interface of electrode and electrolyte. The nanomaterials with high surface area and high porosity are considered as the best performance electrode materials for supercapacitors. Consequently, the synthesis and the capacitive characterization of the high surface area nanomaterials such as nanotubes,6 nanowires,7 etc., have been carried out extensively in the past few years. Among these nanostructures, conducting networks of nanowires is considered promising for supercapacitor electrodes because of their distinctive characteristics of conducting pathways, surface interactions, and nanoscale dimensions.
The cost effectiveness and ease of synthesis are the important factors in the successful commercialization of supercapacitors. For these reasons, polymers such as polyaniline have been considered as the most promising materials for electrode material in the redox supercapacitors.8 Up to now, most of the methods used to prepare polyaniline nanostructures are indirect and need sophisticated techniques to apply the nanostructured polyaniline onto a substrate for practical applications.9–12 Nanostructured polyaniline, with different morphologies, has been synthesized using various techniques such as template synthesis,9 self-assembly,10 emulsions,11 and interfacial polymerization.12 However, such techniques requires relatively large amounts of surfactants, and it is tedious to recycle the surfactants after polymerization. This drawback can be overcome by directly depositing the polyaniline nanowires on the substrate without the involvement of the surfactants.13 Moreover, polyaniline nanowires prepared by such a technique can have different properties as compared to indirect methods due to lack of surfactant. Here, we report the excellent supercapacitive properties of large-area networks of polyaniline nanowires, directly deposited on the stainless steel electrode.
Experimental
The aniline and the 
 were obtained from Wako Chemicals. Research grade stainless steel (SS) (grade 304,
were obtained from Wako Chemicals. Research grade stainless steel (SS) (grade 304,  thick) was obtained from the Nilaco Corporation. The SS was polished with emery paper to a rough finish, washed free of emery particles, and then air-dried. An electrochemical cell was assembled in a three-electrode configuration in which the counter electrode was platinum (Pt), the reference electrode was a saturated calomel electrode (SCE), and working electrode was SS. The total deposition area of the SS was
thick) was obtained from the Nilaco Corporation. The SS was polished with emery paper to a rough finish, washed free of emery particles, and then air-dried. An electrochemical cell was assembled in a three-electrode configuration in which the counter electrode was platinum (Pt), the reference electrode was a saturated calomel electrode (SCE), and working electrode was SS. The total deposition area of the SS was  and the separation between Pt and SS was
and the separation between Pt and SS was  . An electrolyte solution of
. An electrolyte solution of  aniline was used for the electrochemical deposition of polyaniline nanowires on the SS electrode. The deposition of polyaniline was carried out at the constant potential of
aniline was used for the electrochemical deposition of polyaniline nanowires on the SS electrode. The deposition of polyaniline was carried out at the constant potential of  for several minutes. This potential was chosen because polyaniline (PANI) changes from the emeraldine (EM) to pernigraniline (PE) form above this potential.14 Subsequent to deposition, the electrode was washed in distilled water and dried in an oven at
for several minutes. This potential was chosen because polyaniline (PANI) changes from the emeraldine (EM) to pernigraniline (PE) form above this potential.14 Subsequent to deposition, the electrode was washed in distilled water and dried in an oven at  for a day. The polyaniline nanowire∕SS electrode was characterized for microstructure, cyclic voltammetry, charge-discharge cycling, and impedance spectroscopy. The electrochemical deposition as well as the characterization was performed using Auto-lab PGSTAT 30 instrument (Eco-chemie, The Netherlands, http:∕∕www.ecochemie.nl) connected to a three-electrode cell. The microstructure and the thickness of the composites were evaluated by means of JEOL field emission scanning electron microscope (FESEM, JEOL, JSM-6340F).
for a day. The polyaniline nanowire∕SS electrode was characterized for microstructure, cyclic voltammetry, charge-discharge cycling, and impedance spectroscopy. The electrochemical deposition as well as the characterization was performed using Auto-lab PGSTAT 30 instrument (Eco-chemie, The Netherlands, http:∕∕www.ecochemie.nl) connected to a three-electrode cell. The microstructure and the thickness of the composites were evaluated by means of JEOL field emission scanning electron microscope (FESEM, JEOL, JSM-6340F).
Results and Discussion
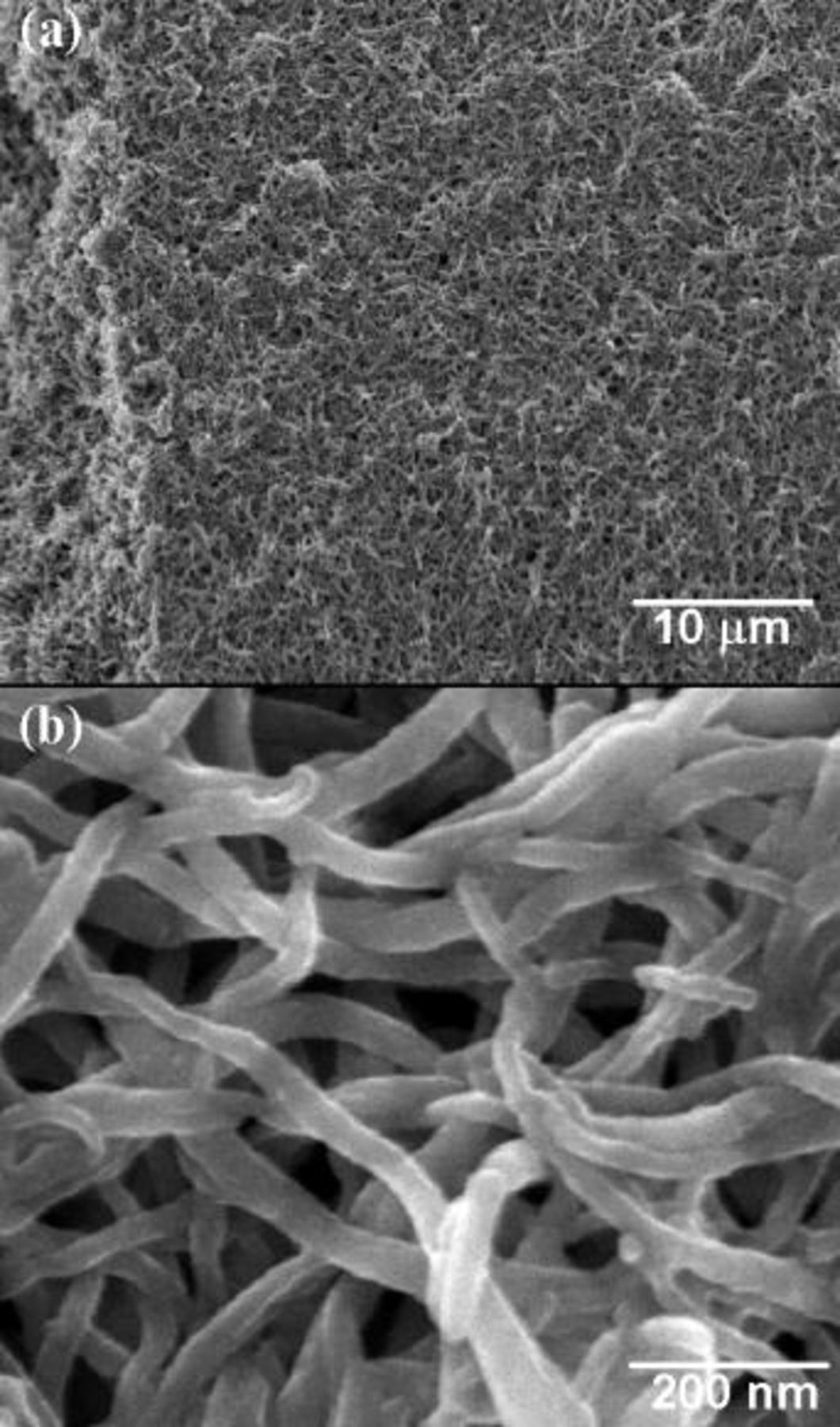
Figure 1a shows the low-resolution SEM image of the polyaniline nanowires network whereas the high resolution SEM image is shown in Fig. 1b. From Fig. 1, it can be inferred that the nanowire network is highly porous and polyaniline nanowires are interconnected. The diameter of the nanowires was in the range of  . The thickness and the size of the film was
. The thickness and the size of the film was  and
and  , respectively. The deposition time was
, respectively. The deposition time was  . To the best of the authors' knowledge, this is the largest synthesized polyaniline nanowire network. Although polyaniline nanowires have been studied for various potential applications such chemical∕electrochemical sensors, actuators, gas-separation membranes, and secondary batteries,15–18 here, such a large area network of polyaniline nanowires has been characterized for supercapacitor applications.
. To the best of the authors' knowledge, this is the largest synthesized polyaniline nanowire network. Although polyaniline nanowires have been studied for various potential applications such chemical∕electrochemical sensors, actuators, gas-separation membranes, and secondary batteries,15–18 here, such a large area network of polyaniline nanowires has been characterized for supercapacitor applications.
Figure 1. (a), (b) SEM images of the polyaniline nanowires at different magnifications.
The specific capacitance values were evaluated by a charge-discharge cycling measurement, which is considered to be the most reliable. Specific capacitance values can be calculated by the following relationship, i.e., specific capacitance  . Here,
. Here,  is the discharge current in amperes,
is the discharge current in amperes,  is the discharge time in seconds corresponding to the voltage difference
is the discharge time in seconds corresponding to the voltage difference  in volts, and
in volts, and  is the electrode mass in grams. The supercapacitive behavior was examined by charge-discharge cycling at various currents densities ranging from
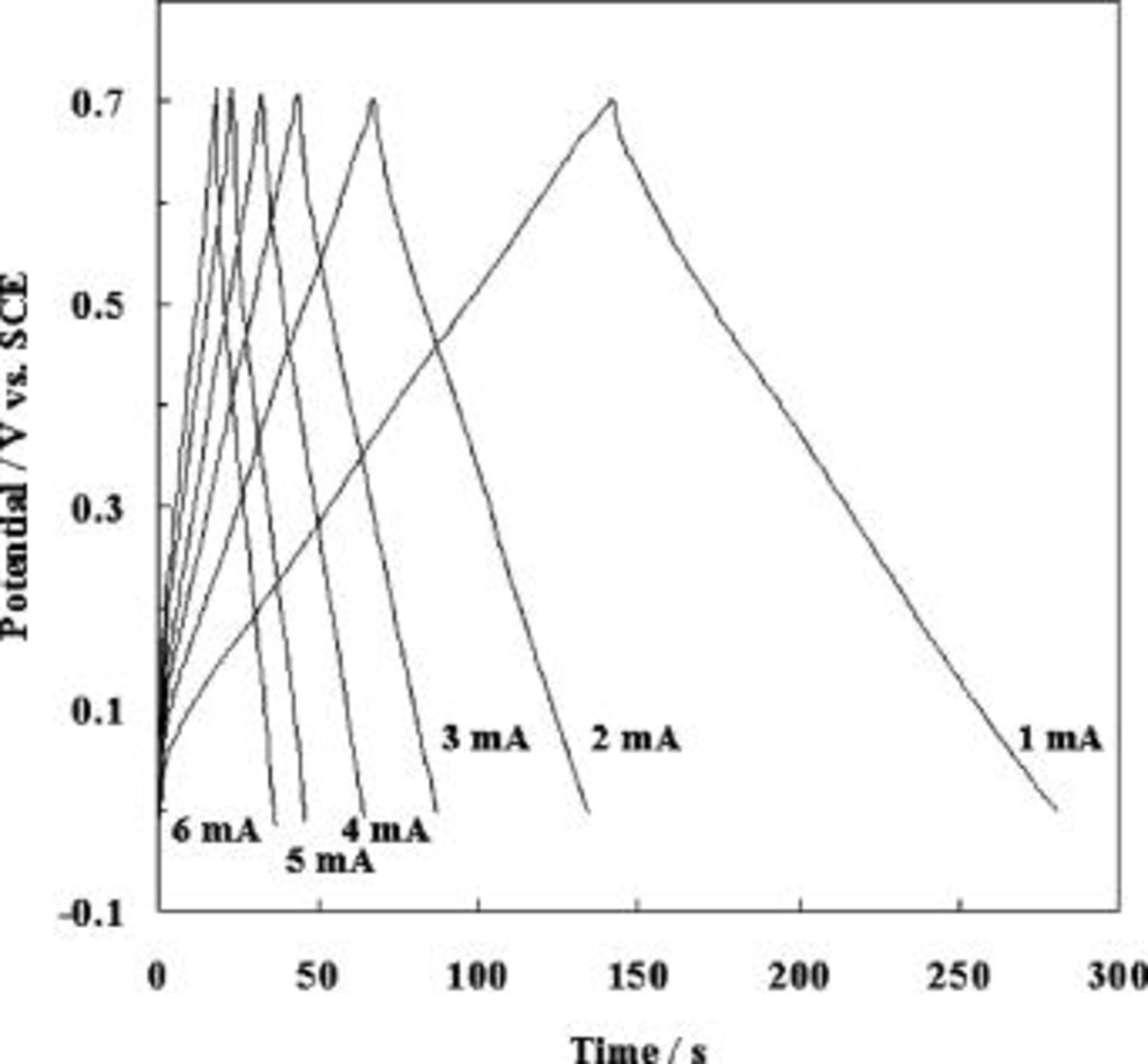
is the electrode mass in grams. The supercapacitive behavior was examined by charge-discharge cycling at various currents densities ranging from  as shown in Fig. 2. The capacitor's potential varies linearly with time during both charging and discharging processes. The efficiency of charge∕discharge cycling is more than 0.99, and there is no IR drop. This trend is continued even at high current density of
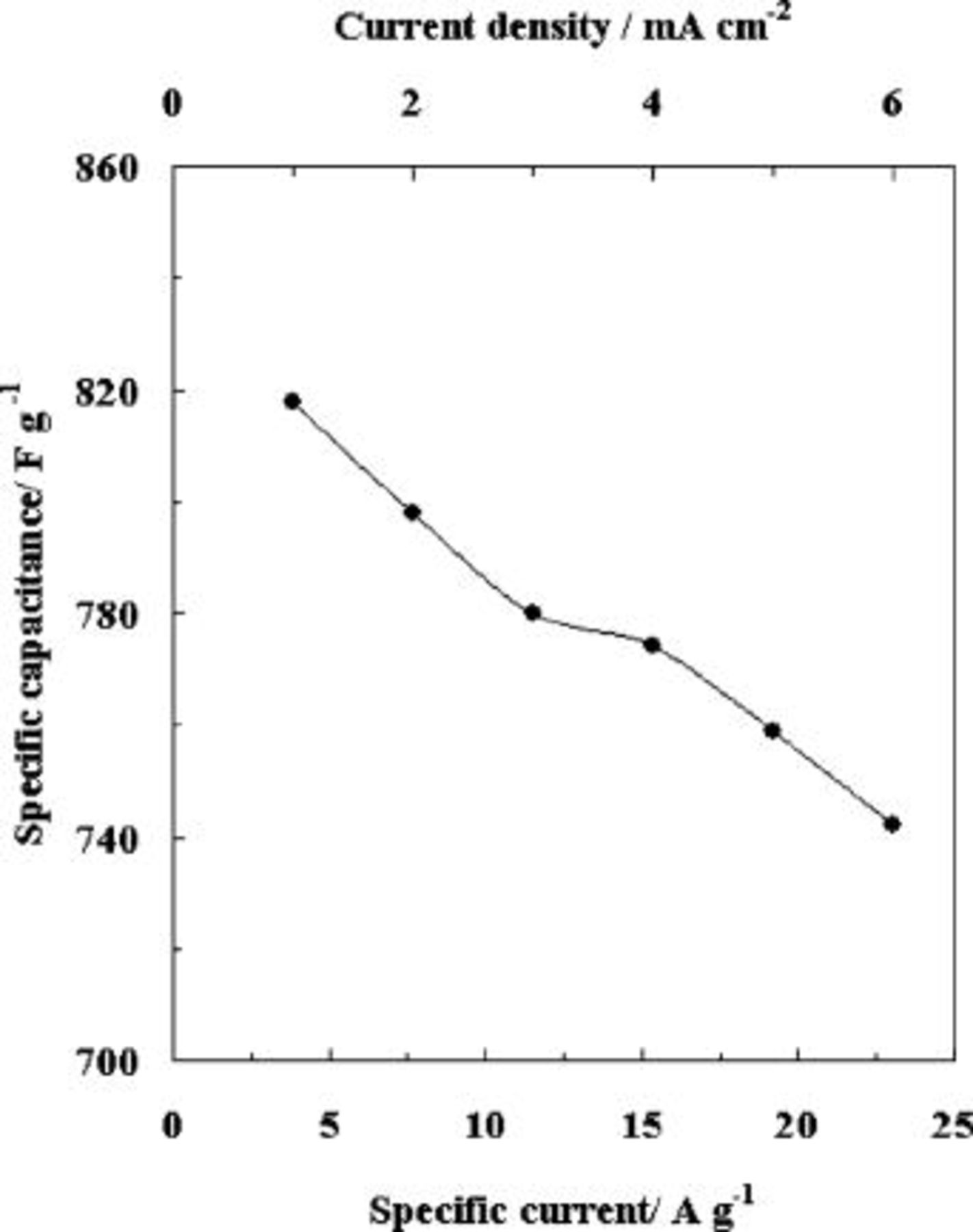
as shown in Fig. 2. The capacitor's potential varies linearly with time during both charging and discharging processes. The efficiency of charge∕discharge cycling is more than 0.99, and there is no IR drop. This trend is continued even at high current density of  , which indicates excellent supercapactive characteristics. Figure 3 shows the calculated specific capacitance (SC) values from charge-discharge curves at various current densities. A high SC value of
, which indicates excellent supercapactive characteristics. Figure 3 shows the calculated specific capacitance (SC) values from charge-discharge curves at various current densities. A high SC value of  was obtained for
was obtained for  current density. The basic redox reaction1
current density. The basic redox reaction1
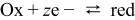
can play a vital role due to the nanowire nature of the polyanilne. The increased contribution of this redox reaction and increased excess of the electrolyte due to the nanoporous nature of the network can lead to very high specific capacitance of the polyaniline nanowires.
Figure 2. Charge∕discharge cycling curves of the polyaniline nanowires at various current densities in 
 electrolyte.
electrolyte.
Figure 3. Relationship of the specific capacitance of the polyaniline nanowire with respect to specific current  and current density
and current density  .
.
The specific capacitance decreases as the current density increases and drops to  for a current density of
for a current density of  . This current density corresponds to a specific current value of
. This current density corresponds to a specific current value of  , and a drop in the specific capacitance value of 9%. There is a 61% drop in the specific capacitance value of the PANI in the case of the non-wire form of PANI,8 when the current is increased from
, and a drop in the specific capacitance value of 9%. There is a 61% drop in the specific capacitance value of the PANI in the case of the non-wire form of PANI,8 when the current is increased from  . In the case of PANI nanowires, the specific capacitance is highly stable even for large current densities. This can be due to the large surface area accessible for redox reactions at the porous network of polyaniline nanowires which is not the case with the non-wire form of polyanilne.8
. In the case of PANI nanowires, the specific capacitance is highly stable even for large current densities. This can be due to the large surface area accessible for redox reactions at the porous network of polyaniline nanowires which is not the case with the non-wire form of polyanilne.8
The specific power (SP) and specific energy (SE) can be calculated from charge-discharge cycling data using the following relationships
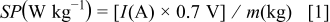
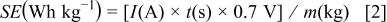
where  ,
,  , and
, and  are the discharge current in amperes, discharge time in seconds, and mass of PANI in kg. Based upon Eq. 1, 2, the calculated
are the discharge current in amperes, discharge time in seconds, and mass of PANI in kg. Based upon Eq. 1, 2, the calculated  and
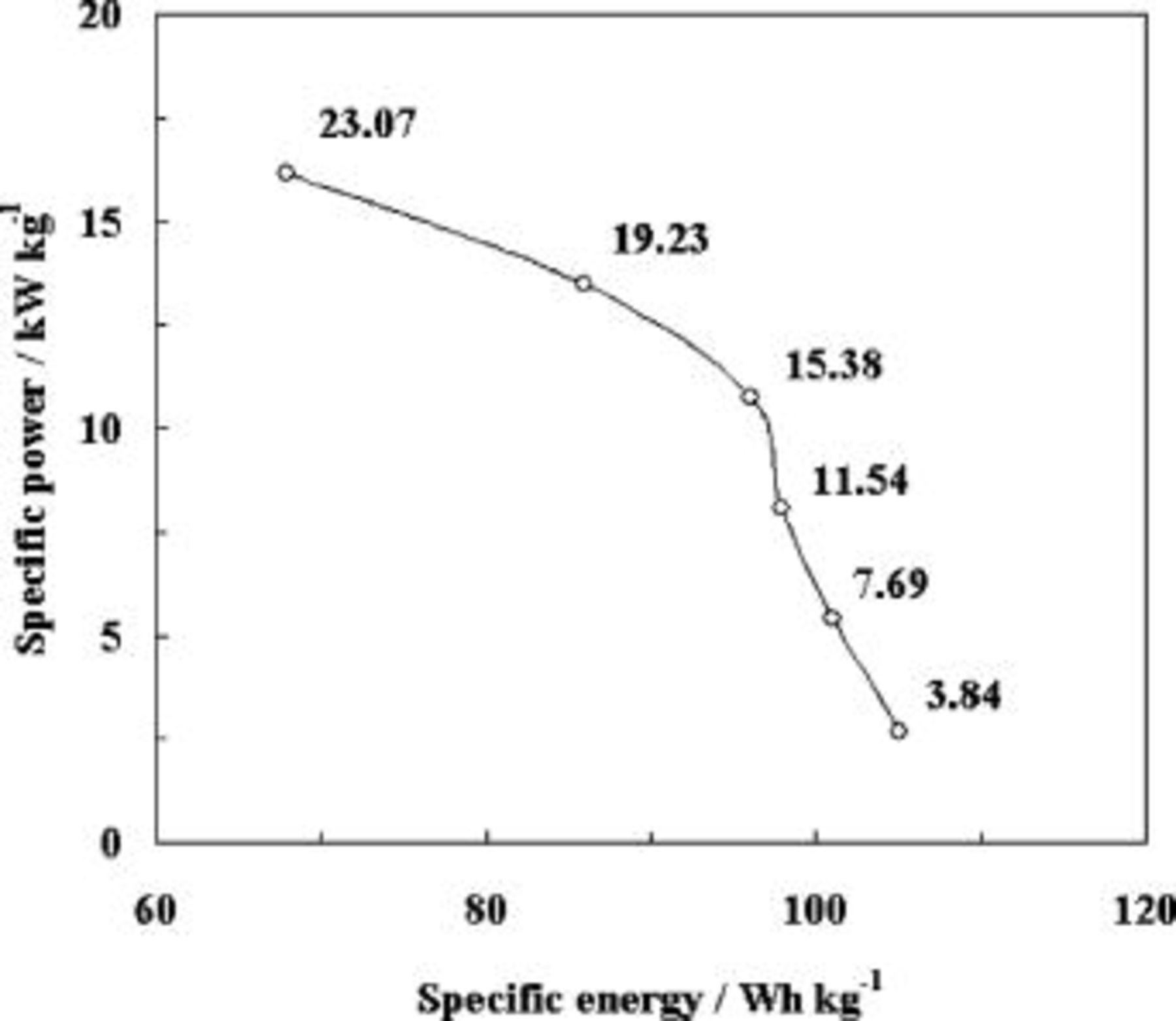
and  values are shown in Fig. 4. At the specific energy of
values are shown in Fig. 4. At the specific energy of  , a specific power of
, a specific power of  was obtained whereas at the specific energy of
was obtained whereas at the specific energy of  , a specific power of
, a specific power of  was obtained, which is several time higher than the
was obtained, which is several time higher than the  specific power obtained at
specific power obtained at  specific energy for the non-wire form of polyaniline deposited by the potentiodynamic method.8 The cycle-life test of polyaniline nanowires was performed at
specific energy for the non-wire form of polyaniline deposited by the potentiodynamic method.8 The cycle-life test of polyaniline nanowires was performed at  for
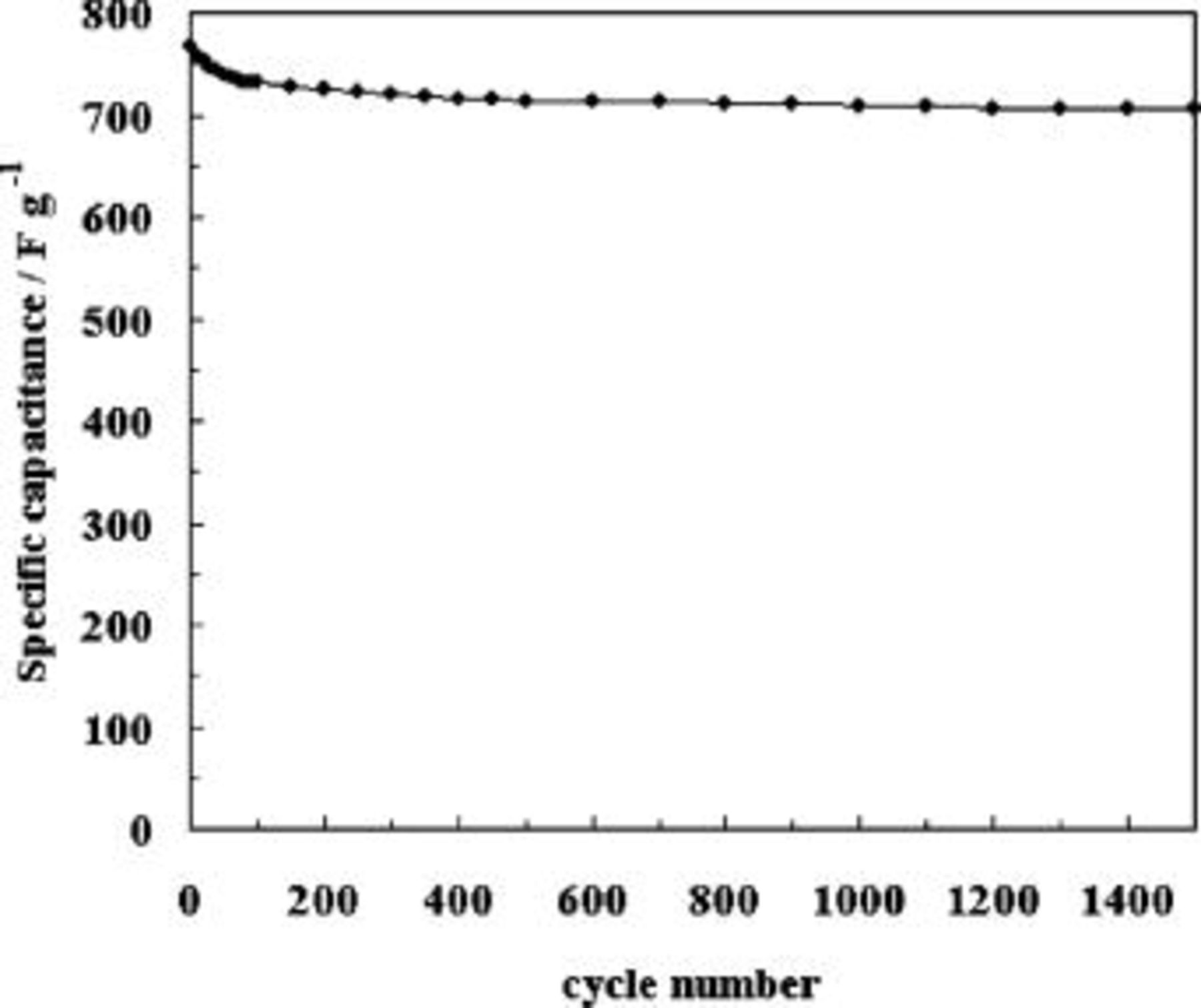
for  . As shown in Fig. 5, there is a small decrease in the specific capacitance value in the first
. As shown in Fig. 5, there is a small decrease in the specific capacitance value in the first  and thereafter the specific capacitance remains almost constant. The decrease in the specific capacitance was 7% in the first
and thereafter the specific capacitance remains almost constant. The decrease in the specific capacitance was 7% in the first  and 1% in the subsequent
and 1% in the subsequent  . This decrease in the capacitance in the present case is much lower compared to the value reported for the nonwire form of PANI.8
. This decrease in the capacitance in the present case is much lower compared to the value reported for the nonwire form of PANI.8
Figure 4. Ragon plot of a polyaniline nanowire capacitor. Charge-discharge specific current (in  ) values are indicated at each data point.
) values are indicated at each data point.
Figure 5. Cyclic-life data of the polyaniline nanowire capacitor. The specific capacitance was calculated for the charge-discharge cycling at the current density of  in
in 
 electrolyte.
electrolyte.
Conclusions
Nanowires of polyaniline were deposited on SS electrode by the potentiostatic method at  vs SCE. A maximium specific capacitance value of
vs SCE. A maximium specific capacitance value of  was obtained at
was obtained at  current density. At the specific energy of
current density. At the specific energy of  , a specific power of
, a specific power of  was obtained. High specific capacitance, specific power, and stability of the material were demonstrated.
was obtained. High specific capacitance, specific power, and stability of the material were demonstrated.
Acknowledgments
This present work was supported by the Japan Science and Technology Agency through the Core Research for Evolutionary Science and Technology program under the project Development of Advanced Nanostructured Materials for Energy Conversion and Storage.
Kyushu University assisted in meeting the publication costs of this article.