Abstract.
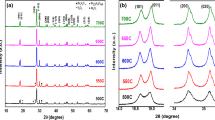
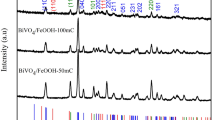
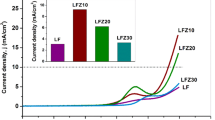
Bismuth vanadate (BiVO4) and its heterostructures with tungstate (WO3) were prepared by solvothermal technique. Phase purity of these compounds was analysed by collecting powder. X-ray diffraction data revealed the co-existence of both BiVO4 and WO3 phases. Transmission electron microscopic measurements on these samples revealed that the average particles sizes of these heterostructures are in the submicron range. Optical band gap is found in the range of 2.30 to 2.45 eV by using UV-visible spectrometer. Optical spectra reveal two distinct absorption edges corresponding to both existing phases. Electrochemical activity of BiVO4 and BiVO4/WO3 heterostructures was studied using electrochemical workstation for applicability of oxygen evolution reaction (OER). These results indicate that the electrochemical activity was improved by forming heterostructures as compared with pristine compounds. From the Tafel slope analysis, it was found that the second electron transfer step is the rate determining step in OER mechanism in BiVO4/WO3 heterostructures.
Similar content being viewed by others
References
Z.-L. Wang, D. Xu, J.-J. Xu, X.-B. Zhang, Chem. Soc. Rev. 43, 7746 (2014)
M. Tahir, L. Pan, F. Idrees, X. Zhang, L. Wang, J.-J. Zou, Z.L. Wang, Nano Energy 3, 136 (2017)
Y. Matsumoto, E. Sato, Mater. Chem. Phys. 14, 397 (1986)
M.-M. Wohlfahrt, J. Heitbaum, J. Electroanal. Chem. Interf. Electrochem. 237, 251 (1987)
M. Musiani, F. Furlanetto, R. Bertoncello, J. Electroanal. Chem. 465, 160 (1995)
C.D. Pauli, S. Trasatti, J. Electroanal. Chem. 538, 145 (2002)
A. Singh, L. Spiccia, Coord. Chem. Rev. 257, 2607 (2013)
I. Katsounaros, S. Cherevko, A.R. Zeradjanin, K.J. Mayrhofer, Angew. Chem. Int. Ed. 53, 102 (2004)
L. Trotochaud, J.K. Ranney, K.N. Williams, S.W. Boettcher, J. Am. Chem. Soc. 134, 17253 (2012)
J.A. Seabold, K.-S. Choi, J. Am. Chem. Soc. 134, 2186 (2012)
J. Yang, D. Wang, X. Zhou, C. Li, Chem. Eur. J. 19, 1320 (2013)
T.W. Kim, K.-S. Choi, Science 343, 990 (2014)
S. Tokunaga, H. Kato, A. Kudo, Chem. Mater. 13, 4624 (2001)
J. Yu, A. Kudo, Adv. Funct. Mater. 16, 2163 (2006)
S.J. Moniz, J. Zhu, J. Tang, Adv. Energy Mater. 4, 1301590 (2014)
D. Eisenberg, H.S. Ahn, A.J. Bard, J. Am. Chem. Soc. 136, 14011 (2014)
J.H. Kim, J.S. Lee, Energy Environ. Focus 3, 339 (2014)
I. Grigioni, K.G. Stamplecoskie, E. Selli, P.V. Kamat, J. Phys. Chem. C 119, 20792 (2015)
P. Chatchai, Y. Murakami, S.-Y. Kishioka, A.Y. Nosaka, Y. Nosaka, Electrochim. Acta 54, 1147 (2009)
Z. Meng, A. Fujii, T. Hashishin, N. Wada, T. Sanada, J. Tamaki, K. Kojima, H. Haneoka, T. Suzuki, J. Mater. Chem. C 3, 1134 (2015)
V. Sivakumar, R. Suresh, K. Giribabu, V. Narayanan, J. Cogent. Chem. 1, 1074647 (2015)
Y. Hu, D. Li, Y. Zheng, W. Chen, Y. He, Y. Shao, X. Fu, G. Xiao, J. Appl. Catal. B: Environ. 104, 30 (2001)
W. Zhou, H. Liu, J. Wang, D. Liu, G. Du, J. Cui, ACS Appl. Mater. Interf. 2, 2385 (2010)
Y. Meng, W. Song, H. Huang, Z. Ren, S.-Y. Chen, S.L. Suib, J. Am. Chem. Soc. 136, 11452 (2014)
E. Guerrini, H. Chen, S. Trasatti, J. Solid State Electrochem. 11, 939 (2007)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
The EPJ Publishers remain neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saraswathi, P., Ramarao, S.D., Kumar, R.A. et al. Solvothermal synthesis of BiVO4/WO3 heterostructures and their applicability towards electrochemical water oxidation reactions. Eur. Phys. J. Plus 134, 186 (2019). https://doi.org/10.1140/epjp/i2019-12629-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/i2019-12629-7