Article Text
Abstract
Background Large-scale mitochondrial DNA deletions (LMD) are a common genetic cause of mitochondrial disease and give rise to a wide range of clinical features. Lack of longitudinal data means the natural history remains unclear. This study was undertaken to describe the clinical spectrum in a large cohort of patients with paediatric disease onset.
Methods A retrospective multicentre study was performed in patients with clinical onset <16 years of age, diagnosed and followed in seven European mitochondrial disease centres.
Results A total of 80 patients were included. The average age at disease onset and at last examination was 10 and 31 years, respectively. The median time from disease onset to death was 11.5 years. Pearson syndrome was present in 21%, Kearns-Sayre syndrome spectrum disorder in 50% and progressive external ophthalmoplegia in 29% of patients. Haematological abnormalities were the hallmark of the disease in preschool children, while the most common presentations in older patients were ptosis and external ophthalmoplegia. Skeletal muscle involvement was found in 65% and exercise intolerance in 25% of the patients. Central nervous system involvement was frequent, with variable presence of ataxia (40%), cognitive involvement (36%) and stroke-like episodes (9%). Other common features were pigmentary retinopathy (46%), short stature (42%), hearing impairment (39%), cardiac disease (39%), diabetes mellitus (25%) and renal disease (19%).
Conclusion Our study provides new insights into the phenotypic spectrum of childhood-onset, LMD-associated syndromes. We found a wider spectrum of more prevalent multisystem involvement compared with previous studies, most likely related to a longer time of follow-up.
- congenital
- hereditary
- and neonatal diseases and abnormalities
- sequence deletion
- paediatrics
- phenotype
- prognosis
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author (KB) upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- congenital
- hereditary
- and neonatal diseases and abnormalities
- sequence deletion
- paediatrics
- phenotype
- prognosis
Introduction
Mitochondrial disease caused by large-scale mitochondrial DNA deletions (LMD) was first described in 1988.1 The deletion is most often sporadic; however, a recurrence risk of 4% in the offspring of affected women has been reported.2 The prevalence of LMD disorders in the adult population has been estimated from 1.2:100 0003 to 1.6:100 000,4 while the point prevalence under 16 years of age was estimated at 1:180 000.5
The majority with LMD-associated syndromes (LMDS) have one of three overlapping phenotypes: Pearson syndrome (PS), Kearns-Sayre syndrome (KSS) or progressive external ophthalmoplegia (PEO).6 There are also reports of LMDS manifesting as a mild myopathy or more atypically with phenotypes similar to Leigh syndrome, MELAS (mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes) or Reye-like syndrome.7–9
PS was originally defined by a combination of sideroblastic anaemia and exocrine pancreas dysfunction10 and is often fatal in infancy.11 Those who survive usually developed KSS.12 Subsequent studies showed that the PS phenotype is more complex with multiple organ system involvement.13 KSS is a progressive multisystem disorder defined classically by the triad of pigmentary retinopathy, external ophthalmoplegia and onset before the age of 20 years, with one or more additional features including cardiac conduction block, cerebrospinal fluid protein concentration >100 mg/dL or cerebellar ataxia.14 15 PEO is characterised by progressive ptosis, ophthalmoplegia, oropharyngeal weakness, variably severe proximal limb weakness and absence of a multisystem affection. Patients with PEO and multisystem involvement, while not fulfilling the KSS criteria, have often been described as ‘PEO plus’. Since many patients with LMD have phenotypes that do not strictly match the original criteria for KSS, PEO or PS,9 13 16–19 new criteria for the different phenotypes have been proposed.17
Diagnosis of mitochondrial DNA (mtDNA) deletion syndromes is based on characteristic clinical findings, blood and bone marrow examination (for PS), muscle biopsy abnormalities, decreased activity of oxidative phosphorylation complexes in a tissue sample and genetic confirmation of an LMD. The choice of tissue20 and technique is important, with next-generation DNA sequencing becoming an increasingly useful diagnostic tool.21
Currently, treatment of LMDS is mainly symptomatic. Dietary supplements are given frequently but are of uncertain efficacy.22 There are, however, several emerging treatments,23 24 but assessment of treatment efficacy in clinical trials is difficult due to the complex and heterogeneous phenotypes, variable clinical course and lack of natural history data.22
Most reports of LMDS have been based on individual cases or small case series,9 17 18 25 and very few studies have attempted to describe the full clinical phenotype in a larger cohort of patients and address the question of the clinical course. This is a joint study from the Mitochondrial Clinical and Research Network (MCRN), a network established to facilitate clinical research collaboration among mitochondrial disease centres. The aim of this study is to describe the phenotypic spectrum and outcome in 80 patients with childhood-onset LMDS and a long follow-up time.
Methods
Study design and population
This retrospective study was conducted in seven MCRN centres from five countries: Sweden (Gothenburg, Stockholm), Denmark (Copenhagen), the Netherlands (Maastricht), Finland (Oulu, Helsinki) and Norway (Bergen). The major inclusion criteria were a genetically verified large-scale (>1.1 kb) mtDNA deletion considered to be pathogenic and disease onset before 16 years of age (online supplemental figure 1). No patients with multiple LMDs were included.
Supplemental material
Patient data were collected using an electronic case report form in a centrally administered database, similar to the network’s earlier studies on Leigh syndrome and polymerase gamma (POLG) related diseases.26 27 The data included family history; medical history and survival status; clinical, biochemical, histological, genetic and neuroimaging findings; and treatments received.
Patients were classified according to Mancuso et al 17 into ‘KSS spectrum’, defined as LMD with ptosis and/or ophthalmoparesis and at least one of retinopathy, ataxia, cardiac conduction defects, hearing loss, failure to thrive/short stature, cognitive impairment, tremor or cardiomyopathy; PS, defined as LMD with refractory anaemia; and PEO, defined as LMD with ptosis and/or ophthalmoparesis that did not fulfil the KSS spectrum or the PS criteria.
Statistical analysis
Statistical analyses were performed using SAS V.9.4 and MedCalc V.19.8. Due to the rarity of the disease, the study was not powered for specific statistical hypotheses and the statistical evaluations were considered exploratory.
Results
Demographics and family history
A total of 80 patients matched the inclusion criteria, 48 females and 32 males. None was genetically related and 96% of patients were of Caucasian descent. All patients were sporadic without a family history of an LMD.
Genetic findings
The median time between disease onset and genetic confirmation of the diagnosis was 11.0 (range 0–63.1) years. Due to differences in methodology, the exact deletion breakpoints were known for 36 (45%) patients, while only deletion length was available for the remaining 44 (55%). The deletion length ranged from approximately 2000 bp to 9557 bp.
Deletion heteroplasmy level was known for 49 patients, and 48 analyses were performed on skeletal muscle tissue, 14 analyses on peripheral blood, 4 analyses on urine sediment, 4 times on cavum oris epithelium, 3 times on liver tissue, 2 times on orbicularis oculi muscle, and 1 time each in bone marrow, fibroblasts, kidney tissue and duodenal tissue.
In three of the patients who had peripheral blood analysed, the deletion was not detected (all three with KSS spectrum phenotype), and for one patient (PEO) it was not detected in urine sediment. Analyses of urine sediment (one KSS spectrum, three PEO), cavum oris epithelium (one KSS spectrum, three PEO) and duodenal tissue (PS) showed consistently low heteroplasmy levels, ranging from 0% to 10%, while skeletal muscle analysis at the same time showed markedly higher levels, ranging from 9% to 75%.
Biochemical, morphological and enzyme histochemical findings
Respiratory chain enzyme activities in muscle mitochondria were abnormal in 25 of 42 patients (60% of assayed patients), with isolated complex I deficiency seen in 8, isolated complex IV deficiency in 2, isolated complex III deficiency in 1 and combined deficiencies in 12 patients, while detailed data were not available for 2 patients (online supplemental table 1).
Supplemental material
Muscle histology indicative of mitochondrial disease was seen in 52 of 56 patients (93% of patients with known data), with ragged-red fibres in 38, cytochrome c oxidase deficiency in 37 and abnormal mitochondrial proliferation in 14 patients.
Of patients with known data, elevated lactate levels in blood and cerebrospinal fluid were seen in 32 of 50 and 9 of 15 patients, respectively.
Elevated CSF protein levels were found in 17 of 20 patients, 11 with KSS spectrum, 5 with PS and 1 with PEO.
Major clinical phenotypes
Using the revised criteria for LMDS,17 patients were categorised into three groups of phenotype: 23 had ptosis and/or ophthalmoplegia without additional KSS spectrum symptoms (PEO group), 40 patients without PS fulfilled the KSS spectrum criteria, while 17 patients were categorised as PS (table 1). Eight patients with PS later met the criteria for KSS spectrum.
Clinical features of 17 patients with Pearson syndrome
In the KSS spectrum group (table 2), there were varying degrees of multisystem involvement, with one patient affected by symptoms from nine systems. Involvement of two to five systems was seen in 31 of 40 (78%) patients.
Overview of organ involvement in patients with KSS spectrum
Even though the patients in the PEO group did not have additional KSS spectrum symptoms as defined by Mancuso et al,17 there were additional clinical features besides limb myopathy: type 2 diabetes mellitus (T2DM) (1 of 23), depression (1 of 23) and unspecified neuropsychiatric disorder (1 of 23).
Disease course
The median age at disease onset was 10.3 years (range 0–15.4) (online supplemental figure 2). The median time from disease onset to last follow-up was 19.4 years (range 0.0–61.4). The median age at last evaluation was 28.6 years (range 3.0–75.7). The most common clinical features at onset were ptosis (60%), PEO (44%) and anaemia (17%) (online supplemental table 2).
Supplemental material
Supplemental material
The distribution of clinical features according to age of onset is shown in figure 1. In patients with disease onset up to 10 years of age (35 of 80 patients), anaemia was the most prevalent clinical feature. In later onset disease, ophthalmological disorders were the most common. Major clinical features that were typically seen at disease onset, but comparatively rare later in the disease course, included anaemia, ptosis and failure to thrive. Conversely, cardiac conduction block, hearing impairment, visual impairment, renal disorder, diabetes mellitus, ataxia, pigmentary retinopathy, learning disability, muscular atrophy, limb weakness and dysphagia more often appeared later during the disease course.
Onset age in years of major clinical features. Patients for which a precise feature onset date is not known are excluded. Within each box, the horizontal lines denote the median values; boxes extend from the 25th to the 75th percentile of each group’s distribution of values; vertical extending lines denote adjacent values (ie, the most extreme values within 1.5 IQR of the 25th and 75th percentile of each group); dots denote observations outside the range of adjacent values. PEO, progressive external ophthalmoplegia.
Initial patient presentation was preceded by infection in 7 of 42 patients with known data. Acute exacerbations were experienced by 16 of 61 patients. The most common cause of exacerbations was infection (9 of 16 patients). Hospitalisation due to acute exacerbation was required at least once by 12 of 16 patients.
Two patients had anaemia onset at birth. Both had intrauterine growth retardation and were born small for gestational age at term. One of them had additional pathological signs at birth, including microcephaly, hypotonia, proximal limb weakness, respiratory involvement and hyperlactataemia, while the other had no other pathological features at birth. Only one other patient was reported small for gestational age.
Survival
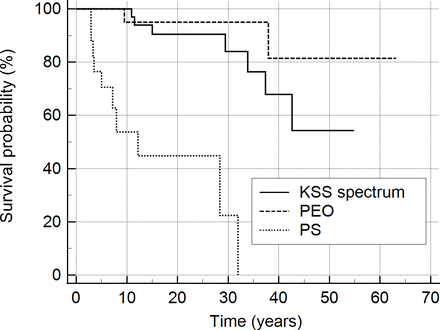
Based on the last known status at the time of data recording for each patient, 60 patients were alive, 19 had died and 1 was lost to follow-up. The median age at death was 18.9 years (range 3.3–55.6 years). The median time elapsed from disease onset to death was 11.5 years (range 2.9–42.6 years). The Kaplan-Meier survival probability since disease onset was markedly decreased for the PS group compared with PEO and KSS spectrum (log-rank test p<0.0001; figure 2). There was no significant gender difference in survival.
Kaplan-Meier analysis of survival since disease onset for patients of the KSS spectrum (n=40), PEO (n=23) and PS (n=17) groups. KSS, Kearns-Sayre syndrome; PEO, progressive external ophthalmoplegia; PS, Pearson syndrome.
Out of the 19 deceased patients, 10 belonged to the PS group, 7 to the KSS spectrum and 2 to the PEO group. The causes of death were identified for 10 patients, and in all these cases it was due to disease progression, with multiorgan failure being the most frequently reported cause. The causes of death for the two patients in the PEO group were not known; they were both male, one in their early 20s with widened QRS complexes but no other known cardiac disorder and one in their early 50s with no known cardiac disorder.
Haematological features
Childhood-onset transfusion-requiring anaemia was found in 17 patients. Sideroblastic anaemia was found in nine patients and exocrine pancreatic insufficiency in five. Fourteen patients had anaemia at disease onset, while in two patients there was more than 2 years between the first signs of disease until they developed anaemia. Multisystem morbidity and mortality were high in this group (table 1). At the time of last follow-up, anaemia persisted in 7 of these patients, while 10 were free from haematological involvement.
Neurological, neuromuscular and psychiatric features
Skeletal muscle weakness was reported in 42 of 74 patients, and limb muscle weakness was found in 41 of them. One patient had bilateral weakness of the sternocleidomastoid muscles without any associated limb weakness. Muscular atrophy was seen in 21 of 50, muscular hypotonia in 11 of 73 and exercise intolerance was reported in 15 of 60 patients. Fatigue was reported in 26 of 69 patients.
Psychomotor retardation was present in 11 patients and learning disability in 18 patients. A psychiatric disorder, predominantly of a depressive nature, was present in six of the patients.
Cerebellar ataxia was reported in 25 of 63 patients, while 4 of 75 had spasticity, 1 of 63 had dystonia and 5 of 74 had unspecified limb hypertonia. Peripheral neuropathy was reported in three patients.
Epileptic seizures were reported in four patients, of whom two were seizure-free with anticonvulsant treatment at last follow-up. Stroke-like episodes were reported in 7 patients (5 in the KSS spectrum group and 2 in the PS group), while 11 patients had migraine and/or cyclic vomiting.
Neuroimaging
Of the 80 patients, 29 underwent brain MRI at a median time of 6.6 years (range 0.5–46.2) after clinical onset. Abnormal findings were present in 18 of 29 (62%), as detailed in table 3 and online supplemental figure 3, with 1 patient having unspecified abnormal findings. Abnormal MRI findings in 17 patients where specified findings were known included signal abnormalities in the globus pallidus in 53%, in supratentorial white matter in 47%, in the cerebellar white matter, thalamus, mesencephalon and pons respectively in 24%, in other basal ganglia and medulla respectively in 12%, and in the cerebral cortex in 6%. Cerebral or cerebellar atrophy was detected in 29% of the patients.
Supplemental material
Brain MRI findings
Eyes and hearing
The most frequent ophthalmological manifestation was ptosis, affecting 70 patients. It was unilateral in five of them. PEO was observed in 66 patients and was bilateral in all but one of them. In one patient, the ptosis and ophthalmoplegia were reversible and were only seen during intercurrent infections at a younger age. Pigmentary retinopathy was detected in 37 of the patients and visual impairment was found in 31 of the patients. Strabismus was seen in 16, optic atrophy in 4 and nystagmus in 1 of the patients. Corneal manifestations including keratoconus, oedema, dystrophy and haze were reported in five patients.
Hearing impairment was reported in 31 patients. For all patients with known data (n=21), the type of impairment was sensorineural. Use of hearing aid was reported in 17 patients.
Cardiac involvement
Cardiac disorder was reported in 31 patients. Conduction block was present in 25 patients, with second-degree atrioventricular block in 3, third-degree atrioventricular block in 8, bifascicular block in 7, and right bundle branch block and partial left bundle branch block in 1 each. Fourteen patients were treated with pacemaker. More uncommon features included supraventricular/sinus tachycardia (n=2), dilated cardiomyopathy (n=1), hypertrophic cardiomyopathy (n=1), sinus bradycardia (n=1), wide QRS complex (n=1), premature ventricular contractions (n=1), heart failure (n=1), aortic valve insufficiency (n=1) and bicuspid aortic valve (n=1). One patient died of cardiac arrest.
Endocrine system and growth involvement
Diabetes mellitus was present in 20 patients. Fourteen patients developed type 1 diabetes mellitus (T1DM), with an age of onset ranging from 0.1 years to 34.4 years, while six patients developed T2DM with an age of onset ranging from 1.9 years to 57.4 years (figure 1).
Eleven of 20 male patients and 9 of 28 female patients were short of stature (height less than 2 SD below the mean for age and sex). Growth hormone (GH) deficiency was seen in nine patients (age at onset: 0.8–15.8 years), and seven of them were short of stature, while growth data were not available for the remaining two.
Five patients developed hypoparathyroidism during the disease course, with an age at onset ranging from 3.8 to 17.5 years. Four patients developed hypothyroidism during the disease course with an age at onset ranging from 4.5 years to 46 years. One patient developed Addison’s disease.
Visceral and gastrointestinal tract involvement
Renal manifestations were reported in 15 patients (10 of 17 with PS, 5 of 48 with KSS spectrum), with isolated tubulopathy being the most common (n=9). In two patients, it was diagnosed as Fanconi syndrome. Combined tubular and glomerular involvement was reported in four patients and isolated glomerular disease was seen in one patient. Mild liver involvement, without signs of liver failure, was reported in nine patients. Five patients had exocrine pancreatic dysfunction and all these also had anaemia. Dysphagia was reported in 18 of 62 patients. Chronic constipation and chronic diarrhoea were seen in five patients each.
Discussion
While LMD is one of the most frequent genetic causes of mitochondrial disease and has been known for more than 30 years,1 10 14 considerable delay in establishing the diagnosis remains common. We found a median time from onset to diagnosis of 11 years. Further, the clinical phenotypes of PS, KSS and PEO classically associated with LMD appear insufficient in describing the entire spectrum of the disease.6 Our study confirms that these diseases appear to be more of a continuum. Prior to our study, knowledge of the clinical spectrum of these diseases has been limited. Most information was contained in small case series, with few reports attempting to describe the full clinical phenotype in larger cohorts of patients spanning all ages,9 17 25 apart from one relatively large study of 34 patients18 which focused on patients with paediatric onset. Our study of 80 patients with paediatric disease onset provides detailed information about the clinical features and outcome. The male to female ratio in our cohort of 0.67 is similar to two earlier studies,9 17 while other studies18 25 have described an even gender distribution.
The diagnostics is not always straightforward. The mtDNA deletion was occasionally absent in blood cells, while the lactate levels in blood and CSF were only inconsistently elevated. Our finding of low heteroplasmy levels in urine sediment analysis is consistent with what has been recently reported.20 The most reliable biomarker for disease detection was morphological findings indicating mitochondrial myopathy in those who underwent a muscle biopsy.
We identified 17 patients with childhood-onset anaemia, of whom only 5 had additional exocrine pancreatic insufficiency compatible with the original criteria for the Pearson marrow-pancreas syndrome.10 Since the first reports of PS, the phenotypic spectrum has gradually been shown to be more diverse than initially described.18 28–30 Our patients confirm this phenotypic diversity, with all exhibiting multisystem involvement. Eight of the patients with PS later fulfilled the criteria for KSS spectrum disorder. Two of the patients with PS in our study had a prenatal onset, which has only rarely been reported before.11 30 It is important to include PS in the differential diagnoses of refractory anaemia in neonates and infants with or without accompanying signs of exocrine pancreatic dysfunction, especially in the presence of other organ involvement.
Skeletal muscle is the most frequently involved organ system in LMDS. In our study we found major involvement of the extraocular muscles, with ptosis in 88% and external ophthalmoplegia in 82%. Ptosis and external ophthalmoplegia are defining clinical features of LMDS and the prevalence increases with age, as illustrated by an even higher prevalence of 94% in the study from Japan.9 The mechanism behind the preferential involvement of extraocular muscles is likely to be a more sustained metabolic demand than other skeletal muscles, with more COX-deficient fibres and a lower mutational threshold compared with skeletal muscles.31 Compared with a previous paediatric study,18 we found a much higher prevalence of external ophthalmoplegia. Exercise intolerance is an important feature of myopathy in LMDS and was reported in 25% of patients. In a previous paediatric study,18 the presence of exercise intolerance was not reported. It is essential to search for the presence of exercise intolerance in patients suspected to have LMDS as it is an indicator of an associated mitochondrial myopathy.
The presence of central nervous system involvement, for example, ataxia, cognitive impairment, psychiatric involvement, stroke-like episodes and migraine, was higher in our cohort than reported in earlier studies.17 18 25 Stroke-like episodes were reported in one patient in the Japanese study and only rarely previously in LMDS.32 33 Stroke-like episodes are more typical in MELAS and POLG-related mitochondrial disease, where they frequently associate with seizures.34 In contrast, epilepsy in LMDS is typically rare and treatable and was only found in 5% in our study.
We report a large spectrum of MRI changes associated with LMD. In contrast to a recent study that identified abnormal MRI of the brain in all patients,35 we found normal MRI in 38% of our patients. Characteristic MRI findings included signal abnormalities in supratentorial white matter and globus pallidus. The increased T2 signalling from white matter possibly represents spongiform degeneration, while the occasional finding of increased T1 signalling from the basal ganglia could be secondary to iron accumulation, as has been described in previous neuropathological studies.36
Ophthalmological features, predominantly pigmentary retinopathy and visual impairment, were also more frequent in our study compared with the previous paediatric study.18 Retinal photoreceptors have very high requirements for ATP production and retinal involvement is a frequent finding in both primary and secondary mitochondrial diseases.37 Hearing impairment was also a more common finding. The initial site of lesion for hearing loss in LMDS seems to be the cochlea, indicating that even in the presence of radiological evidence of brain abnormality involving the auditory pathway, the diagnosis, in general, does not represent a contraindication to cochlear implantation.38
Cardiac involvement was found in 39% of our patients. The most frequent cardiac finding was a conduction defect, identified in 31%, and most of these patients were treated with pacemaker. None of our patients was reported to have had ventricular arrhythmias, and only one patient in our study died from sudden death. In a study of 35 children and adults with KSS, a mortality of 11% was found, all due to sudden cardiac death.39 The reason for our different results is unclear, but it seems that cardiac manifestations could be a major determinant of outcome in LMDS and it should be monitored closely and treated appropriately. A weakness of our data is that the cause of death was not specified for nine patients (47% of the deceased patients).
Endocrine system or growth involvement was common, the most frequent involvements being short stature or diabetes mellitus. GH deficiency was identified in nine patients, but unfortunately we do not have data on GH treatment in these patients. Previous reports suggested heterogeneous response to GH treatment, with occasional short-term increase in growth velocity, but often with poor long-term effect.40 A possible reason could be that the associated short stature is instead caused by respiratory chain dysfunction in the growth plate cartilage.41 Diabetes mellitus in mitochondrial disease frequently has an insidious onset due to gradual reduction of the insulin production caused by slow destruction of the beta cells, as opposed to insulin resistance.42 The prevalence of diabetes mellitus in our study is similar to a previous paediatric study18 and much higher than the 11% reported in a British study.43 Mitochondrial dysfunction’s role in T1DM development has been suggested to act both through enhancing autoimmune susceptibility in beta cells and upregulating T cell autoreactive activity.44
Renal manifestations were more common than reported in a previous paediatric study,18 while it was not reported in two other studies.9 17 In two-thirds the renal manifestation was tubular and in one-third glomerular. Proximal tubulopathy, and especially De Toni-Debré Fanconi syndrome, is known to be comparably frequent in LMDS.45 Renal manifestations may occasionally be the first manifestation of a mitochondrial disease, but are more frequently detected later in the course and may be overshadowed by extrarenal manifestations46 and the frequency is probably underestimated. We identified mild hepatopathy without effect on liver function in 11% and swallowing difficulties in 29% of our patients. Liver failure seems to be a rare manifestation in LMDS but has been described in early-onset severe forms with a PS phenotype,30 47 while dysphagia is important to actively look for since it could lead to aspiration, malnutrition and secondary deterioration.
One limitation of the current study is its retrospective and multicentre design, which occasionally makes it difficult to access clinical data at a desired level of detail. We feel that this is partially balanced by the large number of patients and multiple centre involvement, although differing clinical practices and methods of investigations among the centres may still contribute a confounding factor to be considered when interpreting the results. Furthermore, we recognise that the co-occurrence of some clinical features otherwise common in the population can be incidental findings. A strength of the study was the long follow-up observation time compared with previous studies, which could explain the higher prevalence of neurological involvement found in our study.
To our knowledge this is the largest study on LMDS in the paediatric population. The diagnostic delay identified in our study suggests a possible underdiagnosis of the disease. An improved understanding and awareness of the clinical spectrum is therefore important. Compared with previous studies, we found a broader spectrum of more frequent multiorgan involvement. The progressive course and the wide range of organ system involvement demonstrate the need for coordinated multidisciplinary follow-up of these patients for timely diagnosis and treatment of the different possible organ manifestations.
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author (KB) upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
Our study complied with the ethical guidelines and was conducted in accordance with the World Medical Association Declaration of Helsinki. The study protocol was approved by the Ethics Committee in Gothenburg, Sweden (Dnr 289–17). Each participating centre received ethical approval and complied with the requirements of their local regulatory authorities.
Acknowledgments
This work was generated within MCRN (Mitochondrial Clinical and Research Network) and the European Reference Network for hereditary metabolic disorders (MetabERN). We would like to thank Qualitis for electronic data capture services and statistical support.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors KB and ND designed the study. KB was responsible for overseeing data collection and analysis. KB and ND conceptualised, drafted and revised the manuscript. All authors participated in data acquisition, manuscript revision and approval of the final manuscript. KB and ND are the guarantors of the manuscript.
Funding The study was supported by the Queen Silvia Children's Hospital Research Foundation (KB, ND, MT), the Swedish state under an agreement between the Swedish government and the country councils (ND: ALFGBG-718681; MT: ALFGBG-427421), and the Gothenburg Society of Medicine (KB). IFMdC is funded by NeMO (nr. 20_P10).
Competing interests KM has received support for attending meetings and travel from Nordic Infucare and Biogen Finland. IFMdC has received consulting fees from Reneo Pharma.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.