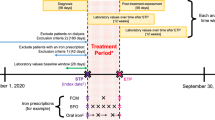
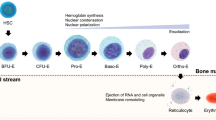
Abstract—
End-stage renal disease (ESRD) is associated with a variety of erythron alterations including structural and functional changes in circulating erythrocytes (RBCs), which are potential risk factors to cause RBC malfunctions and corresponding anemia of different severity. However, the mechanisms of such changes in RBCs remain understudied. Here we used flow cytometry to estimate intracellular esterase activity, phosphatidylserine externalization, and reticulocyte count and state, laser diffraction for osmotic and ammonium stress tests, and spectrophotometry to evaluate the generation of unstable hemoglobin (Hb) forms in RBCs of patients with ESRD before and after the hemodialysis (HD) session. RBCs in ESRD were more osmotically fragile and had an increased swelling rate in the ammonium stress test. HD did not affect RBC deformability but led to Hb oxidation to ferryl forms and triggered such apoptosis-like events, as a decrease in intracellular esterase activity and an increase in the number of annexin-V-positive cells. Our data indicate that uremic syndrome, combined with the mechanical and chemical effects of HD therapy, challenges the erythron of HD patients and contributes to a multifactorial decrease in RBC function and aggravation of renal anemia.





Similar content being viewed by others
REFERENCES
Glassock R.J. 2008. Uremic toxins: What are they? An integrated overview of pathobiology and classification. J. Ren. Nutr. 18 (1), 2–6.
Vos F.E., Schollum J.B., Coulter C.V., Doyle T.C., Duffull S.B., Walker R.J. 2011. Red blood cell survival in long-term dialysis patients. Am. J. Kidney Dis. 58 (4), 591–598.
Kliche K., Gerth U., Pavenstädt H., Oberleithner H. 2015. Recharging red blood cell surface by hemodialysis. Cell Physiol. Biochem. 35 (3), 1107–1115.
Bonan N.B., Steiner T.M., Kuntsevich V., Virzì G.M., Azevedo M., Nakao L.S., Barreto F.C., Ronco C., Thijssen S., Kotanko P., Pecoits-Filho R., Moreno-Amaral A.N. 2016. Uremic toxicity-induced eryptosis and monocyte modulation: The erythrophagocytosis as a novel pathway to renal anemia. Blood Purif. 41 (4), 317–323.
Fishbane S., Spinowitz B. 2018. Update on anemia in ESRD and earlier stages of CKD: Core curriculum 2018. Am. J. Kidney Dis. 71 (3), 423–435.
Georgatzakou H.T., Tzounakas V.L., Kriebardis A.G., Velentzas A.D., Papageorgiou E.G., Voulgaridou A.I., Kokkalis A.C., Antonelou M.H., Papassideri I.S. 2017. Pathophysiological aspects of red blood cells in endstage renal disease patients resistant to recombinant human erythropoietin therapy. Eur. J. Haematol. 98 (6), 590–600.
Tozoni S.S., Dias G.F., Bohnen G., Grobe N., PecoitsFilho R., Kotanko P., Moreno-Amaral A.N. 2019. Uremia and hypoxia independently induce eryptosis and erythrocyte redox imbalance. Cell Physiol. Biochem. 53, 794–804.
Ayala A., Muñoz M.F., Argüelles S. 2014. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 360438.
Sindhu R.K., Ehdaie A., Farmand F., Dhaliwal K.K., Nguyen T., Zhan C.D., Roberts C.K., Vaziri N.D. 2005. Expression of catalase and glutathione peroxidase in renal insufficiency. Biochim. Biophys. Acta. 1743 (1–2), 86–92.
Gao C., Xie R., Yu C., Ma R., Dong W., Meng H., Zhang Y., Si Y., Zhang Z., Novakovic V., Zhang Y., Kou J., Bi Y., Li B., Xie R., Gilbert G.E., Zhou J., Shi J. 2015. Thrombotic role of blood and endothelial cells in uremia through phosphatidylserine exposure and microparticle release. PLoS One. 10 (11), e0142835.
Bonomini M., Sirolli V., Merciaro G., Antidormi T., Di Liberato L., Brummer U., Papponetti M., Cappelli P., Di Gregorio P., Arduini A. 2005. Red blood cells may contribute to hypercoagulability in uraemia via enhanced surface exposure of phosphatidylserine. Nephrol. Dial. Transplant. 20 (2), 361–366.
Luo J.F., Li J.H., Nie J.J., Li P.P., Zhang H.D., Ma Y.J. 2019. Effect of hemodialysis on the red blood cell life span in patients with end-stage kidney disease. Ther. Apher. Dial. 23 (4), 336–340.
Scherer A., Günther O.P., Balshaw R.F., Hollander Z., Wilson-McManus J., Ng R., McMaster W.R., McManus B.M., Keown P.A. 2013. Alteration of human blood cell transcriptome in uremia. BMC Med. Genomics. 6, 23.
Antonelou M.H., Kriebardis A.G., Velentzas A.D., Kokkalis A.C., Georgakopoulou S.C., Papassideri I.S. 2011. Oxidative stress-associated shape transformation and membrane proteome remodeling in erythrocytes of end stage renal disease patients on hemodialysis. J. Proteomics. 74 (11), 2441–2452.
Huisjes R., Bogdanova A., van Solinge W.W., Schiffelers R.M., Kaestner L., van Wijk R. 2018. Squeezing for life – Properties of red blood cell deformability. Front. Physiol. 9, 656.
McMahon T.J. 2019. Red blood cell deformability, vasoactive mediators, and adhesion. Front. Physiol. 10, 1417.
Orbach A., Zelig O., Yedgar S., Barshtein G. 2017. Biophysical and biochemical markers of red blood cell fragility. Transfus. Med. Hemother. 44 (3), 183–187.
Kikuchi Y., Koyama T., Koyama Y., Tozawa S., Arai T., Horimoto M., Kakiuchi Y. 1982. Red blood cell deformability in renal failure. Nephron. 30 (1), 8–14.
Barns S., Balanant M.A., Sauret E., Flower R., Saha S., Gu Y. 2017. Investigation of red blood cell mechanical properties using AFM indentation and coarse-grained particle method. Biomed. Eng. Online. 16 (1), 140.
Bowry S.K., Gatti E. 2011. Impact of hemodialysis therapy on anemia of chronic kidney disease: The potential mechanisms. Blood Purif. 32 (3), 210–219.
Tugusheva F.A., Zubina I.M. 2009. Oxidative stress and its participation in nonimmune mechanisms of progressing chronic kidney disease. Nephrology. 13 (3), 42–48.
Dutka P. 2008. Guarding against hidden hemolysis during dialysis: An overview. Nephrol. Nurs. J. 35, 45.
Ahmadmehrabi S., Tang W.H.W. 2018. Hemodialysisinduced cardiovascular disease. Semin. Dial. 31 (3), 258–267.
Depond M., Henry B., Buffet P., Ndour P.A. 2020. Methods to investigate the deformability of RBC during malaria. Front. Physiol. 10, 1613.
Sohn M., Lee J.E., Ahn M., Park Y., Lim S. 2021. Correlation of dynamic membrane fluctuations in red blood cells with diabetes mellitus and cardiovascular risks. Sci. Rep. 11 (1), 7007.
Bratosin D., Estaquier J., Petit F., Arnoult D., Quatannens B., Tissier J.P., Slomianny C., Sartiaux C., Alonso C., Huart J.J., Montreuil J., Ameisen J.C. 2001. Programmed cell death in mature erythrocytes: A model for investigating death effector pathways operating in the absence of mitochondria. Cell Death. Differ. 8, 1143–1156.
Riley R.S., Ben-Ezra J.M., Goel R., Tidwell A. 2001. Reticulocytes and reticulocyte enumeration. J. Clin. Lab. Anal. 15 (5), 267–294.
Mindukshev I.V., Sudnitsyna J.S., Skverchinskaya E.A., Andreyeva A.Yu., Dobrylko I.A., Senchenkova E.Yu., Krivchenko A.I., Gambaryan S.P. 2019. Erythrocytes’ reactions to osmotic, ammonium, and oxidative stress are inhibited under hypoxia. Biochem. (Moscow), Supp. Series A: Membr. Cell Biol. 13 (4), 352–364.
Sudnitsyna J.S., Skvertchinskaya E.A., Dobrylko I.A., Nikitina E.R., Krivchenko A.I., Gambaryan S.P., Mindukshev I.V. 2016. Human erythrocyte ammonium transport is mediated by functional interaction of ammonium (RhAG) and anion (AE1) transporters. Biochem. (Moscow), Supp. Series A: Membr. Cell Biol. 10 (4), 301–310.
Sudnitsyna J., Skverchinskaya E., Dobrylko I., Nikitina E., Gambaryan S., Mindukshev I. 2020. Microvesicle Formation induced by oxidative stress in human erythrocytes. Antioxidants (Basel). 9 (10), 929.
Kanias T., Acker J.P. 2010. Mechanism of hemoglobininduced cellular injury in desiccated red blood cells. Free Radic. Biol. Med. 49 (4), 539–547.
Ly J., Marticorena R., Donnelly S. 2004. Red blood cell survival in chronic renal failure. Am. J. Kidney Dis. 44, 715–719.
Koma Y., Onishi A., Matsuoka H., Oda N., Yokota N., Matsumoto Y., Koyama M., Okada N., Nakashima N., Masuya D., Yoshimatsu H., Suzuki Y. 2013. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS One. 8 (11), e80240.
Evans E.A., Hochmuth R.M. 1976. Membrane viscoelasticity. Biophys. J. 16 (1), 1–11.
Bruce L.J., Beckmann R., Ribeiro M.L., Peters L.L., Chasis J.A., Delaunay J., Mohandas N., Anstee D.J., Tanner M.J. 2003. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood. 101 (10), 4180–4188.
Borisov Yu.A., Sudnitsyna J.S., Vlasov L.V., Dulneva L.V., Abolmasov V.O., Mindukshev I.V., Smirnov A.V. 2020. Uremic syndrome triggers red blood cell deformability alteration in hemodialysis patients. Rossiiski Fiziologich. Zhurnal (Rus.). 106 (8), 1025–1040.
Evans B.A., Ansari A.K., Kamyszek R.W., Salvagno M., Welsby J., Fuller M., Welsby I. 2021. Modulation of red blood cell oxygen affinity with a novel allosteric modifier of hemoglobin is additive to the Bohr effect. Blood Cells Mol. Dis. 87, 102520.
Jensen F.B. 2009. The dual roles of red blood cells in tissue oxygen delivery: Oxygen carriers and regulators of local blood flow. J. Exp. Biol. 212 (Pt. 21), 3387–3393.
Chintagari N.R., Jana S., Alayas A.I. 2016. Oxidized ferric and ferryl forms of hemoglobin trigger mitochondrial dysfunction and injury in alveolar type I cells. Am. J. Respir. Cell Mol. Biol. 55, 288–298.
Pantaleo A., Ferru E., Pau M.C., Khadjavi A., Mandili G., Mattè A., Spano A., De Franceschi L., Pippia P., Turrini F. 2016. Band 3 erythrocyte membrane protein acts as redox stress sensor leading to its phosphorylation by p (72) Syk. Oxid. Med. Cell Longev. 2016, 6051093.
Sugie J., Intaglietta M., Sung L.A. 2018. Water transport and homeostasis as a major function of erythrocytes. Am. J. Physiol. Heart Circ. Physiol. 314 (5), H1098–H1107.
Jani V.P., Lucas A., Jani V.P., Munoz C., Williams A.T., Ortiz D., Yalcin O., Cabrales P. 2020. Numerical model for the determination of erythrocyte mechanical properties and wall shear stress in vivo from intravital microscopy. Front. Physiol. 10, 1562
Van Avondt K., Nur E., Zeerleder, S. 2019. Mechanisms of haemolysis-induced kidney injury. Nat. Rev. Nephrol. 15, 671–692.
Deltombe O., Van Biesen W., Glorieux G., Massy Z., Dhondt A., Eloot S. 2015. Exploring protein binding of uremic toxins in patients with different stages of chronic kidney disease and during hemodialysis. Toxins (Basel). 7 (10), 3933–3946.
Itano H.A., Keitel H.G., Thompson D. 1956. Hyposthenuria in sickle cell anemia: A reversible renal defect. J. Clin. Invest. 35 (9), 998–1007.
Bonomini M., Pieroni L., Ronci M., Sirolli V., Urbani A. 2018. Blood cell proteomics in chronic kidney disease. Open Urology and Nephrology. 11, 28–38.
Mohanty J.G., Nagababu E., Rifkind J. M. 2014. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 5, 84.
Welbourn E.M., Wilson M.T., Yusof A., Metodiev M.V., Cooper C.E. 2017. The mechanism of formation, structure and physiological relevance of covalent hemoglobin attachment to the erythrocyte membrane. Free Radic. Biol. Med. 103, 95–106.
Oldenborg P.A., Zheleznyak A., Fang Y.F., Lagenaur C.F., Gresham H.D., Lindberg F.P. 2000. Role of CD47 as a marker of self on red blood cells. Science. 288 (5473), 2051–2054.
Sudnitsyna J.S., Gambaryan S.P., Krivchenko A.I., Mindukshev I.V. 2018. Ammonia/ammonium influx in human erythrocytes. Biol. Membrany (Rus.). 35(4), 398–402.
ACKNOWLEDGMENTS
The work was supported by the Russian Foundation for Basic Research (project no. 19-315-60 015 to J.S.) and by the State Assignment of Ministry of Science and Higher Education of the Russian Federation (project no. AAAA-A18-118012290371-3 to J.S., E.S., and I.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
The study was approved by the Ethical Committee of the 1st Pavlov St. Petersburg State Medical University (protocol no. 217 of January 17, 2020) and the Ethical Committee of Sechenov Institute of Evolutionary Physiology and Biochemistry (protocol no. 2-02 of February 2, 2021) and conducted in accordance with the Helsinki Declaration.
Additional information
Translated by T. Ruzhnikova
Rights and permissions
About this article
Cite this article
Sudnitsyna, J.S., Skverchinskaya, E.A., Zubina, I.M. et al. Alterations in Erythrocyte Deformability and Functions Associated with End-Stage Renal Disease. Biochem. Moscow Suppl. Ser. A 16, 79–90 (2022). https://doi.org/10.1134/S1990747821060118
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747821060118