Abstract
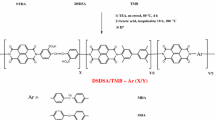
Sulfonated copolymers of 1,3,4-oxadiazole, diphenyl oxide, and 10,10-dioxophenoxathiine are obtained for use as polymer superabsorbents. The one-pot synthesis of the copolymers is carried out in an oleum medium, with the starting reagents being 4,4'-oxydibenzoic acid and hydrazine sulfate. As a result, the copolymers are obtained with the same proportion of the oxadiazole fragment in the chain but different ratio of fragments containing sulfonic acid groups: diphenyl oxide fragments with two sulfonic acid groups and dioxophenoxathiine fragments with one sulfonic acid group and one sulfonyl group. The copolymers are capable of a 100-fold swelling in an aqueous medium and dissolving to a limited extent upon the addition of polar aprotic cosolvents or with an increase in pH. The ratio of sulfonic acid and sulfonyl groups in the composition of the copolymer influences its swelling in water and the viscoelasticity of hydrogels that are in the mesophase state in case of a high content of the copolymer.











Similar content being viewed by others
REFERENCES
S. Bose, T. Kuila, T. X. H. Nguyen, N. H. Kim, K. T. La, and J. H. Lee, Prog. Polym. Sci. 36, 813 (2011).
H. Gao and K. Lian, RSC Adv. 4, 33091 (2014).
M. S. Kamal, A. S. Sultan, U. A. Al-Mubaiyedh, and I. A. Hussein, Polym. Rev. 55, 491 (2015).
T. L. Staple and P. K. Chatterjee, “Synthetic Superabsorbents,” in Absorbent Technology, Ed. by P. K. Chatterjee and B. S. Gupta (Elsevier Science B. V., Amsterdam, 2002), p. 283.
H. Li, Y. Tang, Z. Wang, Z. Shi, S. Wu, D. Song, and Z. Liu, J. Power Sources 178, 103 (2008).
R. Dhodapkar, N. N. Rao, S. P. Pande, T. Nandy, and S. Devotta, React. Funct. Polym. 67, 540 (2007).
R. Sivashankar, A. B. Sathya, K. Vasantharaj, and V. Sivasubramanian, Environ. Nanotechnol., Monit. Manage. 1, 36 (2014).
X. S. Hu, R. Liang, and G. Sun, J. Mater. Chem. A 6, 17612 (2018).
D. Chen, L. Wang, Y. Ma, and W. Yang, NPG Asia Mater. 8, 301 (2016).
X. Wang, S. Lu, L. Chen, J. Li, S. Dai, and X. Wang, J. Radioanal. Nucl. Chem. 306, 497 (2015).
G. Zhao, H. Zhang, Q. Fan, X. Ren, J. Li, Y. Chen, and X. Wang, J. Hazard. Mater. 173, 661 (2010).
D. Shao, G. Hou, J. Li, T. Wen, X. Ren, and X. Wang, Chem. Eng. J. 255, 604 (2014).
B. Hayati, A. Maleki, F. Najafi, H. Daraei, F. Gharibi, and G. McKay, J. Mol. Liq. 224, 1032 (2016).
D. C. Manatunga, R. M. de Silva, K. N. de Silva, and R. Ratnaweera, RSC Adv. 6, 105618 (2016).
K. V. Rao, S. Mohapatra, T. K. Maji, and S. J. George, Chem.-Eur. J. 18, 4505 (2012).
T. Ono, T. Sugimoto, S. Shinkai, and K. Sada, Nat. Mater. 6, 429 (2007).
H. X. Jin, B. Dong, B. Wu, and M. H. Zhou, Polym.- Plast. Technol. Eng. 51, 154 (2012).
M. J. Zohuriaan-Mehr and K. Kabiri, Iran. Polym. J. 17, 451 (2008).
K. Kabiri, H. Omidian, M. J. Zohuriaan-Mehr, and S. Doroudiani, Polym. Compos. 32, 277 (2011).
A. Sannino, A. Esposito, A. D. Rosa, A. Cozzolino, L. Ambrosio, and L. Nicolais, J. Biomed. Mater. Res., Part A 67, 1016 (2003).
S. O. Ilyin, V. G. Kulichikhin, and A. Y. Malkin, Rheol. Acta 55, 223 (2016).
M. Abdulbaki, C. Huh, K. Sepehrnoori, M. Delshad, A. Varavei, J. Pet. Sci. Eng. 122, 741 (2014).
M. J. Zohuriaan-Mehr, H. Omidian, S. Doroudiani, and K. Kabiri, J. Mater. Sci. 45, 5711 (2010).
M. Rikukawa and K. Sanui, Prog. Polym. Sci. 25, 1463 (2000).
J. Roziere and D. J. Jones, Annu. Rev. Mater. Res. 33, 503 (2003).
V. V. Korshak and S. V. Vinogradov, Russ. Chem. Rev. 37, 885 (1968).
S. Janietz and B. Schulz, Eur. Polym. J. 32, 465 (1996).
V. S. Yashchenko, A. A. Pap, Y. V. Matveenko, and V. K. Ol’khovik, Polym. Sci., Ser. B 58, 529 (2016).
D. F. Bairamov, A. E. Chalykh, M. M. Feldstein, R. A. Siegel, N. A. Platé, J. Appl. Polym. Sci. 85, 1128 (2002).
A. Mráček, Int. J. Mol. Sci. 11, 532 (2010).
D. F. Bayramov, P. Singh, G. W. Cleary, R. A. Siegel, A. E. Chalykh, and M. M. Feldstein, Polym. Int. 57, 785 (2008).
S. O. Ilyin, V. V. Makarova, T. S. Anokhina, V. Y. Ignatenko, T. V. Brantseva, A. V. Volkov, and S. V. Antonov, Cellulose 25, 2515 (2018).
A. Malkin, A. Askadsky, A. Chalykh, and V. Kovriga, Experimental Methods of Polymer Physics (Mir, Moscow, 1983).
V. Makarova and V. Kulichikhin, in Interferometry. Research and Applications in Science and Technology, Ed. by I. Padron (InTech, Rijeka, 2012), p. 395.
H. Hatakeyama and T. Hatakeyama, Thermochim. Acta 308, 3 (1998).
A. Mráček, K. Benešová, A. Minařík, P. Urban, and L. Lapčik, J. Biomed. Mater. Res., Part A 83, 184 (2007).
K. Kabiri, H. Omidian, S. A. Hashemi, and M. J. Zohuriaan-Mehr, Eur. Polym. J. 39, 1341 (2003).
O. E. Philippova, Polym. Sci., Ser. C 42, 208 (2000).
H. Omidian, S. A. Hashemi, P. G. Sammes, and I. Meldrum, Polymer 39, 6697 (1998).
P. A. Shabadrov and A. P. Safronov, Polym. Sci., Ser. A 60, 628 (2018).
S. Zhang, H. Chen, S. Liu, and J. Guo, Energy Fuels 31, 1825 (2017).
E. Karadağ, Ö. B. Üzüm, and D. Saraydin, Eur. Polym. J. 38, 2133 (2002).
O. E. Philippova, Y. D. Zaroslov, A. R. Khokhlov, and G. Wegner, Macromol. Symp. 200, 45 (2003).
L. Xu, X. Li, M. Zhai, L. Huang, J. Peng, J. Li, and G. Wei, J. Phys. Chem. B 111, 3391 (2007).
J. Valencia and I. F. Pierola, Eur. Polym. J. 37, 2345 (2001).
R. S. D. Toteja, B. L. Jangida, M. Sundaresan, and B. Venkataramani, Langmuir 13, 2980 (1997).
H. E. Van Wart and G. E. Janauer, J. Phys. Chem. 78, 411 (1974).
C. S. Miner and N. N. Dalton, Glycerol (Reinhold Publ. Corp., New York, 1953).
R. G. LeBel and D. A. I. Goring, J. Chem. Eng. Data 7, 100 (1962).
J. Y. Kim, J. Y. Song, E. J. Lee, and S. K. Park, Colloid Polym. Sci. 281, 614 (2003).
C. P. Kelly, C. J. Cramer, and D. G. Truhlar, J. Phys. Chem. B 110, 16066 (2006).
J. P. Guthrie, Can. J. Chem. 56, 2342 (1978).
A. Malkin, S. Ilyin, T. Roumyantseva, and V. Kulichikhin, Macromolecules 46, 257 (2013).
S. O. Ilyin, V. G. Kulichikhin, and A. Y. Malkin, Polym. Sci., Ser. A 55, 503 (2013).
V. G. Kulichikhin, S. O. Ilyin, M. V. Mironova, A. K. Berkovich, I. E. Nifantyev,and A. Ya. Malkin, Adv. Polym. Technol. 37, 1076 (2018).
S. O. Ilyin, A. Y. Malkin, V. G. Kulichikhin, Y. I. Denisova, L. B. Krentsel, G. A. Shandryuk, A. D. Litmanovich, E. A. Litmanovich, G. N. Bondarenko, and Y. V. Kudryavtsev, Macromolecules 47, 4790 (2014).
A. Malkin, V. Kulichikhin, and S. Ilyin, Rheol. Acta 56, 177 (2017).
Funding
The study was supported by the Russian Foundation for Basic Research (project no. 17-53-04002 Bel_mol_a) and the Belarusian Republican Foundation for Fundamental Research (project no. X17PM-013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yadikova, A.E., Yashchenko, V.S., Makarova, V.V. et al. Synthesis and Properties of Sulfonated Copolymers of Oxadiazole, Dioxophenoxathiine, and Diphenyl Oxide. Polym. Sci. Ser. B 62, 47–60 (2020). https://doi.org/10.1134/S156009042001011X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S156009042001011X