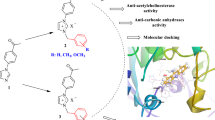
Abstract
1-Phenyl-3-(thiophen-2-yl)-1H-pyrazole-5-carboxamide derivatives were designed and evaluated for their in vitro enzyme inhibitory activities against acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and glutathione S-transferase (GST). In particular, N,1-diphenyl-3-(thiophen-2-yl)-1H-pyrazole-5-carboxamide (10) was found to be the best AChE inhibitor (Ki = 19.88±3.06 µM), [1-phenyl-3-(thiophen-2-yl)-1H-pyrazole-5-yl](piperidin-1-yl)methanone (8) showed the highest inhibitory activity against BChE (Ki = 13.72±1.12 µM), and (morpholin-4-yl)[1-phenyl-3-(thiophen-2-yl)-1H-pyrazole-5-yl]methanone (7) was found to be the best inhibitor for GST (Ki = 16.44±1.58 µM). Molecular docking studies revealed significant interactions at the enzyme active sites, and compounds 7, 8, and 10 exhibited good binding affinities for GST (–9.7 kcal/mol), BChE (–9.4 kcal/mol), and AChE (–9.3 kcal/mol), respectively. The results of the present study have good potential to contribute further structural modifications and pharmacological studies related to enzyme inhibitors.



Similar content being viewed by others
REFERENCES
Elkanzi, N.A.A., J. Chin. Chem. Soc., 2018, vol. 65, no. 2, p. 189. https://doi.org/10.1002/jccs.201700207
Cetin, A., Korkmaz, A., and Kaya, E., Opt. Mater., 2018, vol. 76, p. 75. https://doi.org/10.1016/j.optmat.2017.12.022
Subba Reddy, C.V. and Sumathi, S., ChemistrySelect, 2020, vol. 5, no. 28, p. 8729. https://doi.org/10.1002/slct.202001949
Aljamali, N.M. and Alfatlawi, I.O., Res. J. Pharm. Technol., 2015, vol. 8, no. 9, p. 1225. https://doi.org/10.5958/0974-360X.2015.00224.3
Perin, N., Rep, V., Sović, I., Juričić, Š., Selgrad, D., Klobučar, M., Pržulj, N., Gupta, C.L., Malod-Dognin, N., Pavelić, S.K., and Hranjec, M., Eur. J. Med. Chem., 2020, vol. 185, article no. 111833. https://doi.org/10.1016/j.ejmech.2019.111833
Szabó, T. and Milen, M., Chem. Heterocycl. Compd., 2019, vol. 55, no. 2, p. 126. https://doi.org/10.1007/s10593-019-02427-3
Moore, S., Jaeschke, H., Kleinau, G., Neumann, S., Costanzi, S., Jiang, J.-k., Childress, J., Raaka, B.M., Colson, A., and Paschke, R., Krause, G., Thomas, C.J., abd Gershengorn, M.C., J. Med. Chem., 2006, vol. 49, no. 13, p. 3888. https://doi.org/10.1021/jm060247s
Simoni, D., Roberti, M., Invidiata, F.P., Rondanin, R., Baruchello, R., Malagutti, C., Mazzali, A., Rossi, M., Grimaudo, S., Capone, F., Dusonchet, L., Meli, M., Raimondi, M.V., Landino, M., D’Alessandro, N., Tolomeo, M., Arindam, D., Lu, Sh., and Benbrook, D.M., J. Med. Chem., 2001, vol. 44, no. 14, p. 2308. https://doi.org/10.1021/jm0010320
Tolan, H.E.M., Radwan, M.A.A., Khalaf, H.S., El-Bayaa, M.N., Awad, H.M., and El-Sayed, W.A., Russ. J. Gen. Chem., 2020, vol. 90, no. 8, p. 1544. https://doi.org/10.1134/S1070363220080241
Thakare, P.P., Shinde, A.D., Chavan, A.P., Nyayanit, N.V., Bobade, V.D., and Mhaske, P.C., ChemistrySelect, 2020, vol. 5, no. 15, p. 4722. https://doi.org/10.1002/slct.201904455
Makhanya, T.R., Gengan, R.M., and Kasumbwe, K., ChemistrySelect, 2020, vol. 5, no. 9, p. 2756. https://doi.org/10.1002/slct.201904620
Kevorkyants, R., Emeline, A., and Bahnemann, D., J. Solid State Chem., 2020, vol. 282, article no. 121082. https://doi.org/10.1016/j.jssc.2019.121082
Mirza, A.Z., Nucleosides, Nucleotides Nucleic Acids, 2019, vol. 38, no. 11, p. 836. https://doi.org/10.1080/15257770.2019.1615623
Zengin, G., Sinan, K.I., Ak, G., Mahomoodally, M.F., Paksoy, M.Y., Picot-Allain, C., Glamocilja, J., Sokovic, M., Jekő, J., and Cziáky, Z., Ind. Crops Prod., 2020, vol. 153, article no. 112572. https://doi.org/10.1016/j.indcrop.2020.112572
Türkan, F., Atalar, M.N., Aras, A., Gülçin, İ., and Bursal, E., Bioorg. Chem., 2020, vol. 94, article no. 103333. https://doi.org/10.1016/j.bioorg.2019.103333
Taslimi, P., Köksal, E., Gören, A.C., Bursal, E., Aras, A., Kılıç, Ö., Alwasel, S., and Gülçin, İ., Arab. J. Chem., 2020, vol. 13, no. 3, p. 4528. https://doi.org/10.1016/j.arabjc.2019.10.002
Gülçin, İ., Tel, A.Z., Gören, A.C., Taslimi, P., and Alwasel, S.H., J. Food Meas. Charact., 2019, vol. 13, p. 2062. https://doi.org/10.1007/s11694-019-00127-2
Gao, J., Chen, B., Lin, H., Liu, Y., Wei, Y., Chen, F., and Li, W., Gene, 2020, vol. 743, article no. 144484. https://doi.org/10.1016/j.gene.2020.144484
He, N., Bai, S., Huang, Y., Xing, Y., Chen, L., Yu, F., and Lv, C., Anal. Chem., 2019, vol. 91, no. 8, p. 5424. https://doi.org/10.1021/acs.analchem.9b00713
Gulçin, İ., Taslimi, P., Aygün, A., Sadeghian, N., Bastem, E., Kufrevioglu, O.I., Turkan, F., and Şen, F., Int. J. Biol. Macromol., 2018, vol. 119, p. 741. https://doi.org/10.1016/j.ijbiomac.2018.08.001
Cetin, A., Türkan, F., Taslimi, P., and Gulçin, İ., J. Biochem. Mol. Toxicol., 2019, vol. 33, no. 3, article ID e22261. https://doi.org/10.1002/jbt.22261
Lei, P., Zhang, X., Xu, Y., Xu, G., Liu, X., Yang, X., Zhang, X., and Ling, Y., Chem. Cent. J., 2016, vol. 10, no. 1, p. 1. https://doi.org/10.1186/s13065-016-0186-8
Cutinho, P.F., Roy, J., Anand, A., Cheluvaraj, R., Murahari, M., and Chimatapu, H.V., J. Biomol. Struct. Dyn., 2020, vol. 38, no. 6, p. 1626. https://doi.org/10.1080/07391102.2019.1614094
Aras, A., Bursal, E., Türkan, F., Tohma, H., Kılıç, Ö., Gülçin, İ., and Köksal, E., Chem. Biodiversity, 2019, vol. 16, no. 10, article ID e1900341. https://doi.org/10.1002/cbdv.201900341
Ece, A., J. Biomol. Struct. Dyn., 2020, vol. 38, no. 2, p. 565. https://doi.org/10.1080/07391102.2019.1583606
Taslimi, P., Işık, M., Türkan, F., Durgun, M., Türkeş, C., Gülçin, İ., and Beydemir, Ş., J. Biomol. Struct. Dyn., 2020. https://doi.org/10.1080/07391102.2020.1790422
Bursal, E., Taslimi, P., Gören, A.C., and Gülçin, İ., Biocatal. Agric. Biotechnol., 2020, vol. 28, article no. 101711. https://doi.org/10.1016/j.bcab.2020.101711
Turkan, F., Cetin, A., Taslimi, P., and Gulçin, İ., Arch. Pharm., 2018, vol. 351, no. 10, article no. 1800200. https://doi.org/10.1002/ardp.201800200
Pourabdi, L., Khoobi, M., Nadri, H., Moradi, A., Moghadam, F.H., Emami, S., Mojtahedi, M.M., Haririan, I., Forootanfar, H., and Ameri, A., Eur. J. Med. Chem., 2016, vol. 123, p. 298. https://doi.org/10.1016/j.ejmech.2016.07.043
Kumar, A., Jain, S., Parle, M., Jain, N., and Kumar, P., EXCLI J., 2013, vol. 12, p. 1030. https://doi.org/10.17877/DE290R-7363
Taslimi, P., Turhan, K., Türkan, F., Karaman, H.S., Turgut, Z., and Gulcin, I., Bioorg. Chem., 2020, vol. 97, article no. 103647. https://doi.org/10.1016/j.bioorg.2020.103647
Turkan, F., Cetin, A., Taslimi, P., Karaman, M., and Gulçin, İ., Bioorg. Chem., 2019, vol. 86, p. 420. https://doi.org/10.1016/j.bioorg.2019.02.013
Işık, M., Demir, Y., Kırıcı, M., Demir, R., Şimşek, F., and Beydemir, Ş., Arch. Physiol. Biochem., 2015, vol. 121, no. 3, p. 97. https://doi.org/10.3109/13813455.2015.1026912
Trott, O. and Olson, A., J. Comput. Chem., 2010, vol. 31, no. 2, p. 455. https://doi.org/10.1002/jcc.21334
BIOVIA Discovery Studio Visualizer. https://discover.3ds.com
Hanwell, M.D., Curtis, D.E., Lonie, D.C., Vandermeersch, T., Zurek, E., and Hutchison, G.R., J. Cheminf., 2012, vol. 4, article no. 17. https://doi.org/10.1186/1758-2946-4-17
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
Cetin, A., Türkan, F., Bursal, E. et al. Synthesis, Characterization, Enzyme Inhibitory Activity, and Molecular Docking Analysis of a New Series of Thiophene-Based Heterocyclic Compounds. Russ J Org Chem 57, 598–604 (2021). https://doi.org/10.1134/S107042802104014X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107042802104014X