Abstract
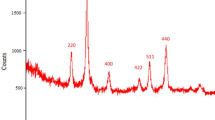
A highly efficient procedure for the one-pot synthesis of polysubstituted pyrrole derivatives by the reaction between of aniline derivatives, β-diketones or β-ketoesters, and β-nitrostyrene derivatives in the presence of Fe3O4@SiO2—CPTMS-guanidine–SO3H as a reusable magnetic nanocatalyst is reported. The magnetic nanocatalyst was prepared and fully characterized by FTIR spectroscopy, scanning electron microscopy, energy dispersive X-ray spectroscopy, X-ray diffraction analysis, thermogravimetric analysis, and vibrating sample magnetometry.
Similar content being viewed by others
References
Reddy, G.R., Reddy, T.R., Joseph, S.C., Reddy, K.S., Reddy, L.S., Kumar, P.M., Krishna, G.R., Reddy, C.M., Rambabu, D., Kapavarapu, R., Lakshmi, C., Meda, T., Priya, K.K., Parsa, K.V.L., and Pal, M., Chem. Commun., 2011, vol. 47, p. 7779. doi https://doi.org/10.1039/C1CC12321A
Yavari, I. and Moradi, L., Tetrahedron Lett., 2006, vol. 47, p. 1627. doi https://doi.org/10.1016/j.tetlet.2005.12.112
Ghabraie, E., Balalaie, S., Bararjanian, M., Bijanzadeh, H.R., and Rominger, F., Tetrahedron, 2011, vol. 67, p. 5415. doi https://doi.org/10.1016/j.tet.2011.05.076
Xu, H., Li, Y., Xing, M., Jia, J., Han, L., Ye, Q., and Gao, J., Org. Lett., 2015, vol. 17, p. 3690. doi https://doi.org/10.1021/acs.orglett.5b01652
Denny, W.A., Rewcastle, G.W., and Baguley, B.C., J. Med. Chem., 1990, vol. 33, p. 814. doi https://doi.org/10.1021/jm00164a054
Bürli, R.W., McMinn, D., Kaizerman, J.A., Hu, W., Ge, Y., Pack, Q., Jiang, V., Gross, M., Garcia, M., Tanaka, R., and Moser, H.E., Bioorg. Med. Chem. Lett., 2004, vol. 14, p. 1253. doi https://doi.org/10.1016/j.bmcl.2003.12.042
Toja, E., Selva, D., and Schiatti, P., J. Med. Chem., 1984, vol. 27, p. 610. doi https://doi.org/10.1021/jm00371a010
Lehuede, J., Fauconneau, B., Barrier, L., Qurakow, M., Piriou, A., and Vierfond, J.M., Eur. J. Med. Chem., 1999, vol. 34, p. 991. doi https://doi.org/10.1016/S0223-5234(99)00111-7
Del Poeta, M., Schell, W.A., Dykstra, C.C., Jones, S., Tidwell, R.R., Czarny, A., Bajic, M., Bajic, M., Kumar, A., Boykin, D., and Perfect, J.R., Antimicrob. Agents Chemother., 1998, vol. 42, p. 2495. doi https://doi.org/10.1128/AAC.42.10.2495
Alizadeh, A., Rezvanian, A., and Bijanzadeh, H.R., Synthesis, 2008, vol. 5, p. 725. doi https://doi.org/10.1055/s-2008-1032168
Maiti, S., Biswas, S., and Jana, U., J. Org. Chem., 2010, vol. 75, p. 1674. doi https://doi.org/10.1021/jo902661y
Alizadeh, A., Bagherinejad, A., Moafi, L., and Zhu, L.G., Synlett., 2016, vol. 27, p. 1803. doi https://doi.org/10.1055/s-0035-1561612
Moradi, L., Piltan, M., and Rostami, H., Chin. Chem. Lett., 2014, vol. 25, p. 123. doi https://doi.org/10.1016/j.cclet.2013.10.009
Ghorbani-Vaghei, R., Sarmast, N., and Mahmoodi, J., Appl. Organometal. Chem., 2016, vol. 31, p. e3681. doi https://doi.org/10.1002/aoc.3681
Shiri, L., Rahmati, S., Ramezani-Nejad, Z., and Kazemi, M., Appl. Organometal. Chem., 2017, vol. 31, p. e3687. doi https://doi.org/10.1002/aoc.3687
Maleki, A., Chalavand, R., and Firouzi Haji, R., Appl. Organometal. Chem., 2017, vol. 32, p. e3916. doi https://doi.org/10.1002/aoc.3916
Maleki, A. and Nooraie-Yeganeh, N., Appl. Organometal. Chem., 2017, vol. 31, p. e3814. doi https://doi.org/10.1002/aoc.3814
Zheng, X., Luo, S., Zhang, L., and Cheng, J.P., Green Chem., 2009, vol. 11, p. 455. doi https://doi.org/10.1039/B823123K
Esmaeili, A. and Kakavand, S., C. R. Chim., 2016, vol. 19, p. 936. doi https://doi.org/10.1016/j.crci.2016.02.009
Ko, S. and Jang, J., Angew. Chem., 2006, vol. 45, p. 7726. doi https://doi.org/10.1002/ange.200602456
Porretta, G., Biava, M., Fioravanti, R., Fischetti, M., Melino, C., Venza, F., Bolle, P., and Tita, B., Eur. J. Med. Chem., 1992, vol. 27, p. 717. doi https://doi.org/10.1016/0223-5234(92)90092-F
Silveira, C.C., Mendes, S.R., Martins, G.M., Schlosser, S.C., Kaufman, T.S., Tetrahedron, 2013, vol. 69, p. 9076. doi https://doi.org/10.1016/j.tet.2013.08.035
Li, B.L., Li, P.H., Fang, X.N., Li, C.X., Sun, J.L., Mo, L.P., and Zhang, Z.H., Tetrahedron, 2013, vol. 69, p. 7011. doi https://doi.org/10.1016/j.tet.2013.06.049
Jagadhane, P.B., Jadhav, N.C., Herlekar, O.P., and Telvekar, V.N., Synth. Commun., 2015, vol. 45, p. 2130. doi https://doi.org/10.1080/00397911.2015.1066392
Jadhav, N.C., Jagadhane, P.B., Patile, H.V., and Telvekar, V.N., Tetrahedron Lett., 2013, vol. 54, p. 3019. doi https://doi.org/10.1016/j.tetlet.2013.04.014
Matloubi Moghaddam, F., Koushki Foroushani, B., and Rezvani, H.R., RSC. Adv., 2015, vol. 5, p. 18092. doi https://doi.org/10.1039/C4RA09348H
Konkala, K., Chowrasia, R., Manjari, P.S., Domingues, N.L.C., and Katla, R., RSC Adv., 2016, vol. 6, p. 43339. doi https://doi.org/10.1039/C6RA08335H
Sarkar, S., Bera, K., Maiti, S., Biswas, S., and Jana, V., Synth. Commun., 2013, vol. 43, p. 1563. doi https://doi.org/10.1080/00397911.2011.650273
Shiri, L., Narimani, H., and Kazemi, M., Appl. Organometal. Chem., 2017, vol. 32, p. e3927. doi https://doi.org/10.1002/aoc.3927
Atashkar, B., Rostami, A., and Tahmasbi, B., Catal. Sci. Technol., 2013, vol. 3, p. 2140. doi https://doi.org/10.1039/C3CY00190C
Acknowledgments
The authors are grateful to the IIam University Research Council for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Russian Text © The Author(s), 2019, published in Zhurnal Organicheskoi Khimii, 2019, Vol. 55, No. 8, pp. 1309.
Rights and permissions
About this article
Cite this article
Rostami, H., Shiri, L. Fe3O4@SiO2—CPTMS—Guanidine—SO3H-catalyzed One-Pot Multicomponent Synthesis of Polysubstituted Pyrrole Derivatives under Solvent-Free Conditions. Russ J Org Chem 55, 1204–1211 (2019). https://doi.org/10.1134/S1070428019080207
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428019080207