Abstract
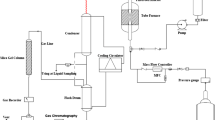
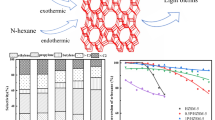
Nanosized ZSM-5 zeolites were synthesized by an in situ seed-induced hydrothermal method, and samples modified with an Fe promoter were prepared by the traditional wet impregnation-thermal decomposition and dielectric barrier discharge plasma (DBD) methods, respectively. The physico-chemical properties of the catalysts were studied by XRD, SEM, TEM, BET, XPS, H2-TPR, NH3-TPD and Py-IR techniques. The catalytic performance was eValuated by the methanol-to-olefin (MTO) reaction. The results showed that the acidity of the catalysts, the dispersity of the Fe promoter and the interaction degree with the ZSM-5 zeolite are closely related to product selectivity in the MTO reaction. Compared with the Fe-NZ5 sample prepared by the traditional impregnation-calcination method, the FeD-NZ5 samples prepared by the DBD method exhibited the higher selectivity of C2—C4 light olefins and the lower coke deposition during long-term eValuation (100 h), which can be attributed to the weaker acid strength, more uniform Fe promoter dispersion and strong interaction with the ZSM-5 zeolite. The developed Fe-modified catalysts have high potential for application in the MTO reaction.
Similar content being viewed by others
References
Tian, P., Wei, Y., Ye, M., and Liu, Z., ACS Catal., 2015, vol. 5, pp. 1922–1938. https://doi.org/10.1021/acscatal.5b00007
Yang, M., Fan, D., and Wei, Y., Adv. Mater., 2019. https://doi.org/10.1002/adma.201902181
Chae, H.J., Song, Y.H., and Jeong, K.E., Kim, C.-U, and Jeong, S.-J., J. Phys. Chem. Solids, 2010, vol. 71, pp. 600–603. https://doi.org/10.1016/jjpcs.2009.12.046
Gao, M., Li, H., and Yang, M., Chem. Eng. J., 2019. https://doi.org/10.1016/j.cej.2018.08.054
Jin, W., Ma, J., and Ma, H., J. Solid State Chem., 2018, vol. 267, pp. 6–12. https://doi.org/10.1016/j.jssc.2018.08.004
Liu, J., Zhang, C., and Shen, Z., Catal. Commun., 2009, vol. 10, pp. 1506–1509. https://doi.org/10.1016/j.catcom.2009.04.004
Hadi, N., Niaei, A., and Nabavi, S.R., J. Taiwan Inst. Chem. Eng., 2016, vol. 59, pp. 173–185. https://doi.org/10.1016/j.jtice.2015.09.017
Yarulina, I., Bailleul, S., and Pustovarenko, A., ChemCatChem, 2016, vol. 8, pp. 3057–3063. https://doi.org/10.1002/cctc.201600650
Velichkina, L.M., Korobitsyna, L.L., and Ulzii, B., Petrol. Chem., 2013, vol. 53, no. 2, pp. 2–121. https://doi.org/10.1134/S0965544113020126
Gorzin, F. and Yaripour, F., Res. Chem. Intermed., 2019, vol. 45, pp. 261–285. https://doi.org/10.1007/s11164-018-3601-z
Magomedova, M.V., Peresypkina, E.G., Davydov, I.A., and Khadzhiev, S.N., Petrol. Chem., 2017, vol. 57, no. 12, pp. 12–1043. https://doi.org/10.1134/S0965544117100115
Jiang, X., Su, X., and Bai, X., Micropor. Mesopor. Mat., 2018, vol. 263, pp. 243–250. https://doi.org/10.1016/j.micromeso.2017.12.029
Rostamizadeh, M. and Yaripour, F., Fuel, 2016, vol. 181, pp. 537–546. https://doi.org/10.1016/j.fuel.2016.05.019
Wang, Z., Zhang, Y., Neyts, E.C., Cao, C., Zhang, X., Ben, W.-L.J., and Liu, C-J., ACS Catal., 2018, vol. 8, pp. 2093–2110. https://doi.org/10.1021/acscatal.7b03723
Liu, C., Li, M., Wang, J., Zhou, X., Guo, Q., Yan, J., and Li, Y., Chin. J. Catal., 2016, vol. 37, no. 3, pp. 3–340. https://doi.org/10.1016/S1872-2067(15)61020-8
Di, L., Zhang, X., Lee, B., et al., Plasma Chem. Plasma Process., 2017, vol. 37, pp. 1535–1549. https://doi.org/10.1007/s11090-017-9834-6
Qi, B., Di, L., Xu, W., and Zhang, X., Lee, B., Lu, P., Ahn, W.-S., and Park, D.-W., J. Mater. Chem. A, 2014, vol. 2, pp. 11885–11890. https://doi.org/10.1039/C4TA02155J
Zhou, R., Rui, N., Fan, Z., and Liu, C.J., Int. J. Hydrogen Energy, 2016, vol. 41, pp. 22017–22025. https://doi.org/10.1016/j.ijhydene.2016.08.093
Jiang, Y., Fu, T., Lü, J., and Li, Z., J. Energy Chem., 2013, vol. 22, pp. 506–511. https://doi.org/10.1016/S2095-4956(13)60066-2
Zhang, K., Kurumov, S.A., Su, X., Snatenkova, Yu.M., Bukina, Z.M., Kolesnichenko, N.V., Wu, W., and Khadzhiev, S.N., Petrol. Chem., 2017, vol. 57, no. 12, pp. 12–1036. https://doi.org/10.1134/S0965544117120179
Cao, X., Zhou, R., Rui, N., Wang, Z., Wang, J., Zhou, X., and Liu, C.-J., Catal. Today, 2017, vol. 297, pp. 219–227. https://doi.org/10.1016/j.cattod.2017.01.042
Leveneur, J., Waterhouse, G.I., Kennedy, J., Metson, J.B., and Mitchell, D.R.G., J. Phys. Chem. C, 2011, vol. 115, pp. 20978–20985. https://doi.org/10.1021/jp206357c
You, Y., Chen, S., Li, J., et al., J. Hazard. Mater., 2020. https://doi.org/10.1016/j.jhazmat.2019.121117
Shi, X., He, H., and Xie, L., Chin. J. Catal., 2015, vol. 36, pp. 649–656. https://doi.org/10.1016/S1872-2067(14)60268-0
Brandenberger, S., Kröcher, O., Casapu, M., Tissler, A., and Althoff, R., Appl. Catal. B-Environ., 2011, vol. 101, pp. 649–659. https://doi.org/10.1016/j.apcatb.2010.11.006
Yang, M., Shao, J., Yang, Z., Yang, H., Wang, X., Wu, Z., and Chen, H., J. Anal. Appl. Pyrolysis., 2019, vol. 137, pp. 259–265. https://doi.org/10.1016/j.jaap.2018.12.003
Bleken, F.L., Chavan, S., Olsbye, U., Boltz, M., Ocampo, F., and Louis, B., Appl. Catal. A-Gen., 2012, vol. 447–448, pp. 178–185. https://doi.org/10.1016/j.apcata.2012.09.025
Yarulina, I., Chowdhury, A.D., Meirer, F., Weckhuysen, B.M., and Gascon, J., Nat. Catal., 2018, vol. 1, pp. 398–411. https://doi.org/10.1038/s41929-018-0078-5
Funding
This work is supported by the National Natural Science Foundation of China (no. 21676074), Intergovernmental International Science and Technology Innovation Cooperation Key Project (2018YFE0108800), the Transformation for Science and Technology Achievements in University of Department of Education of Heilongjiang Province (TSTAU-C2018015)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The other authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, Y., Su, X., Maximov, A.L. et al. Highly Selective MTO Reaction over a Nanosized ZSM-5 Zeolite Modified by Fe via the Low-Temperature Dielectric Barrier Discharge Plasma Method. Russ J Appl Chem 93, 137–148 (2020). https://doi.org/10.1134/S1070427220010152
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427220010152