Abstract
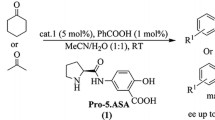
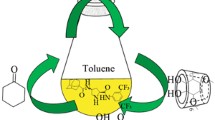
A new organocatalyst trans-4-hydroxy-L-proline-derived calix[4]arene was synthesized and its catalyst performance for the direct asymmetric aldol reactions between cyclohexanone and different aromatic aldehydes was investigated. The effect of a series of reaction conditions such as solvent, water and additives were evaluated in detail, and it was observed that the addition of water had a big effect on the enantioselectivities. Specifically, high anti-diastereoselectivity (anti/syn = 92 : 8) and high enantioselectivity (ee 88%) were obtained from the reaction between cyclohexanone and 4-fluorobenzaldehyde in the presence of water.





Similar content being viewed by others
REFERENCES
Yamashita, Y., Yasukawa, T., Yoo, W.J., Kitanosono, T., and Kobayashi, S., Chem. Soc. Rev., 2018, vol. 47, p. 4388. https://doi.org/10.1039/C7CS00824D
Trost, B.M. and Ito, H., J. Am. Chem. Soc., 2000, vol. 122, 48, p. 12003. https://doi.org/10.1021/ja003033n
Fessner, W-D., in Modern Aldol Reactions, Mahrwald, R., Ed., Berlin: Wiley-VCH, 2004, p. 201.
Tanaka, A. and Barbas, C.F.III., in: Modern Aldol Reactions, Mahrwald, R., Ed., Berlin: Wiley-VCH, 2004, p. 273.
List, B., in Modern Aldol Reactions, Mahrwald, R., Ed., Berlin: Wiley-VCH, 2004, p. 161.
Aydin, A.E., Russ. J. Org. Chem., 2022, vol. 58, p. 820. https://doi.org/10.1134/S1070428022060100
Yanagisawa, A., in Modern Aldol Reactions, Mahrwald, R., Ed., Berlin: Wiley-VCH, 2004, p. 1.
MacMillan, D.W.C., Nature, 2008, vol. 455, p. 304. https://doi.org/10.1038/nature07367
List, B., Lerner, R.A., and Barbas, C.F.III., J. Am. Chem. Soc., 2000, vol. 122, p. 2395. https://doi.org/10.1021/JA994280Y
Mukherjee, S., Yang, J.W., Hoffmann, S., and List, B., Chem. Rev., 2007, vol. 107, p. 5471. https://doi.org/10.1021/cr0684016
Ahrendt, K.A., Borths, C.J., and MacMillan, D.W.C., J. Am. Chem. Soc., 2000, vol. 122, p. 4243. https://doi.org/10.1021/ja000092s
Machajewski, T.D. and Wong, C.H., Angew. Chem., Int. Ed., 2000, vol. 39, p. 1352. https://doi.org/10.1002/(SICI)1521-3773(20000417)39:8<1352::AID-ANIE1352>3.0.CO;2-J
List, B., Acc. Chem. Res., 2004, vol. 37, p. 548. https://doi.org/10.1021/ar0300571
Hajos, Z.G. and Parrish, D.R., German Patent DE 2102623. 1971.
Agami, C., Levisalles, J., and Puchot, C., J. Chem. Soc., Chem. Commun., 1985, p. 441. https://doi.org/10.1039/C39850000441
Bahmanyar, S. and Houk, K.N., J. Am. Chem. Soc., 2001, vol. 123, p. 12911. https://doi.org/10.1021/ja011714s
Bahmanyar, S. and Houk, K.N., J. Am. Chem. Soc., 2001, vol. 123, p. 11273. https://doi.org/10.1021/ja011403h
Clemente, F.R., and Houk, K.N., J. Am. Chem. Soc., 2005, vol. 127, p. 11294. https://doi.org/10.1021/ja0507620
Clemente, F.R. and Houk, K.N., Angew. Chem., Int. Ed., 2004, vol. 43, p. 5765. https://doi.org/10.1002/ange.200460916
Klussmann, M., White, A.J.R., Armstrong, A., and Blackmond, D.G., Angew. Chem., Int. Ed., 2006, vol. 45, p. 7985. https://doi.org/10.1002/anie.200602520
List, B., Hoang, L., and Martin, H.J., Proc. Nat. Acad. Sci. USA, 2004, vol. 101, p. 5839. https://doi.org/10.1073/pnas.0307979101
Marquez, C. and Metzger, J.O., Chem. Commun., 2006, p. 1539. https://doi.org/10.1039/B518288C
Bahmanyar, S., Houk, K.N., Martin, H.J., and List, B., J. Am. Chem. Soc., 2003, vol. 125, p. 2475. https://doi.org/10.1021/ja028812d
Kobayashi, S., Pure Appl. Chem., 2007, vol. 79, p. 235. https://doi.org/10.1351/pac200779020235
Mase, N., Nakai, Y., Ohara, N., Yoda, H., Takabe, K., Tanaka, F., and Barbas, C.F.III., J. Am. Chem. Soc., 2006, vol. 128, p. 734. https://doi.org/10.1021/ja0573312
Mase, N., Watanabe, K., Yoda, H., Takabe, K., Tanaka, F., and Barbas, C.F.III., J. Am. Chem. Soc., 2006, vol. 128, p. 4966. https://doi.org/10.1021/ja060338e
Aratake, S., Itoh, T., Okano, T., Nagae, N., Sumiya, T., Shoji, M., and Hayashi, Y., Chem. Eur. J., 2007, vol. 13, p. 10246. https://doi.org/10.1002/chem.200700363
Guizzetti, S., Benaglia, M., Raimondi, L., and Celentano, G., Org. Lett., 2007, vol. 9, p. 1247. https://doi.org/10.1021/ol070002p
Aratake, S., Itoh, T., Okano, T., Usui, T., Shoji, M., and Hayashi, Y., Chem. Commun., 2007, p. 2524. https://doi.org/10.1039/B702559A
Maya, V., Raj, M., and Singh, V.K., Org. Lett., 2007, vol. 9, p. 2593. https://doi.org/10.1021/ol071013l
Huang, J., Zhang, X., and Armstrong, D.W., Angew. Chem., Int. Ed., 2007, vol. 46, p. 9073. https://doi.org/10.1002/anie.200703606
Zhao, J.-F., He, L., Jiang, J., Tang, Z., Cun, L.-F., and Gong, L.-Z., Tetrahedron Lett., 2008, vol. 49, p. 3372. https://doi.org/10.1016/j.tetlet.2008.03.131
Hayashi, Y., Angew. Chem., 2006, vol. 118, p. 8281. https://doi.org/10.1002/ange.200603378
Blackmond, D.G., Armstrong, A., Coombe, V., and Wells, A., Angew. Chem., 2007, vol. 46, p. 3798. https://doi.org/10.1002/anie.200604952
Hayashi, Y., Sumiya, T., Takahashi, J., Gotoh, H., Urishima, T., and Shoji, M., Angew Chem, Int. Ed., 2006, vol. 45, p. 958. https://doi.org/10.1002/anie.200502488
Emma, M.C., Tamburrini, A., Martinelli, A., Lombardo, M., Quintavalla, A., and Trombini, C., Catalysts, 2020, vol. 10, p. 649. https://doi.org/10.3390/catal10060649
Obregon, A., Milán, M., and Juaristi, E., Org. Lett., 2017, vol. 19, p. 1108. https://doi.org/10.1021/acs.orglett.7b00129
Hayashi, Y., Aratake, S., Okano, T., Takahashi, J., Sumiya, T., and Shoji, M., Angew. Chem., Int. Ed., 2006, vol. 45, p. 5527. https://doi.org/10.1002/anie.200601156
Giacalone, F., Gruttadauria, M., Agrigento, P., Meo, P.L., and Noto, R., Eur. J. Org. Chem., 2010, p. 5696. https://doi.org/10.1002/ejoc.201000913
An, Y.-J., Zhang, Y.-X., Wua, Y., Liu, Z.-M., Pi, C., and Tao, J.-C., Tetrahedron: Asymmetry, 2010, vol. 21, p. 688. https://doi.org/10.1016/j.tetasy.2010.04.019
Eymur, S., Taşcı, E., Uyanık, A., and and Yılmaz, M., Turk. J. Chem., 2020, vol. 44, p. 1278. https://doi.org/10.3906/kim-2003-36
Eymur, S., Akceylan, E., Sahin, O., Uyanik, A., and Yilmaz, M., Tetrahedron, 2014, vol.70, p. 4471. https://doi.org/10.1016/j.tet.2014.05.034
Uyanik, A., Bayrakci, M., Eymur, S., and Yilmaz, M., Tetrahedron, 2014, vol. 70, p. 9307. https://doi.org/10.1016/j.tet.2014.10.063
Akceylan, E., Uyanik, A., Eymur, S., Sahin, O., and Yilmaz, M., Appl. Catal. (A), 2015, vol. 499, p. 205. https://doi.org/10.1016/j.apcata.2015.04.018
Sahin, O., Eymur, S., Uyanik, A., Akceylan, E., and Yilmaz, M., Polycyclic Aromat. Compd., 2018, vol. 38, p. 168. https://doi.org/10.1080/10406638.2016.1176058
Shabir, G., Arif, M., Saeed, A., and Hussain, G., Russ. J. Gen. Chem., 2019, vol. 89, p. 813. https://doi.org/10.1134/S1070363219040285
Burilov, V.A., Mironova, D.A., Grygoriev, I.A., Valiyakhmetova, A.M., Solovieva, S.E., and Antipin, I.S., Russ. J. Gen. Chem., 2020, vol. 90, p. 99. https://doi.org/10.1134/S1070363220010156
Joseph, R., and Rao, C.P., Chem. Rev., 2011, vol. 111, p. 4658. https://doi.org/10.1021/cr1004524
Gutsche, C.D., and Iqbal, M., Org. Syn., 1990, vol. 68, p. 234. https://doi.org/10.15227/orgsyn.068.0234
Collins, E.M., McKervey, M.A., Madigan, E., Moran, M.B., Owens, M., Ferguson, G., and Harris, S.J., J. Chem. Soc., Perkin Trans. 1, 1991, vol.12, p. 3137. https://doi.org/10.1039/P19910003137
Hoang, L., Bahmanyar, S., Houk, K.N., and List, B., J. Am. Chem. Soc., 2003, vol. 125, p. 16. https://doi.org/10.1021/ja028634o
Tang, Z., Jiang, F., Cui, X. L., Gong, Z., Mi, A.Q., Jiang, Y.Z., and Wu, Y.D., Proc. Natl. Acad. Sci. U.S.A., 2004, vol. 101, p. 5755. https://doi.org/10.1073/pnas.0307176101
Lam, Y.H., Houk, K.N., Scheffler, U., and Mahrwald, R., J. Am. Chem. Soc., 2012, vol.134, p. 6286. https://doi.org/10.1021/ja2118392
Fu, A.P., Zhao, C.Y., Li, H.L., Tian, F.H., Yuan, S.P., Duan, Y.B., and Wang, Z.H., J. Phys. Chem. A, 2013, vol. 117, p. 2862. https://doi.org/10.1021/jp3126363
Armstrong, A., Boto, R.A., Dingwall, P., Contreras-Garcia, J., Harvey, M.J., Masona, N.J., and Rzepa, H.S., Chem. Sci., 2014, vol. 5, p. 2057. https://doi.org/10.1039/C3SC53416B
Allemann, C., Gordillo, R., Clemente, F.R., Cheong, P.H.Y., and Houk, K.N., Acc. Chem. Res., 2004, vol. 37, p. 558. https://doi.org/10.1021/ar0300524
Fu, A.P., List, B., and Thiel, W., J. Org. Chem., 2006, vol.71, p. 320. https://doi.org/10.1021/jo052088a
Arino, J.R., Carvajal, M.A., Chaumont, A., and Masia, M., Chem. Eur. J., 2012, vol. 18, p. 15868. https://doi.org/10.1002/chem.201200007
Bahmanyar, S., Houk, K.N., Martin, H.J., and List, B., J. Am. Chem. Soc., 2003, vol. 125, p. 2475. https://doi.org/10.1021/ja028812d
Gutsche, C.D., Dhawan, B., No, K.H., and Muthukrishnan, R., J. Am. Chem. Soc., 1981, vol. 103, p. 3782. https://doi.org/10.1021/ja00403a028
D’Elia, V., Zwicknagl, H., and Reiser., O., J. Org. Chem., 2008, vol. 73, p. 3262. https://doi.org/10.1021/jo800168h
Chen, F., Huang, S., Zhang, H., Liu, F., and Peng. Y., Tetrahedron, 2008, vol. 64, p. 9585. https://doi.org/10.1016/j.tet.2008.07.051
Maya, V., Raj, M., and Singh., V.K., Org. Lett., 2007, vol. 9, p. 2593. https://doi.org/10.1021/ol071013l
Fu, S.D., Fu, X.K., Zhang, S.P., Zou, X.C., and Wu, X.J., Tetrahedron: Asym., 2009, vol. 20, p. 2390. https://doi.org/10.1016/j.tetasy.2009.09.019
Funding
Financial supports by The Scientific and Technological Research Council of Türkiye (Grant no. TBAG-112T349), and Scientific Research Projects Foundation of Selcuk University (SUBAP) are acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
No conflict of interest was declared by the authors.
Supplementary information
Rights and permissions
About this article
Cite this article
Uyanik, A., Sahin, O., Akceylan, E. et al. Effect of Calix[4]arene as a Hydrophobic Substituent on Proline Catalysis of Direct Asymmetric Aldol Reactions in the Presence of Water. Russ J Gen Chem 92, 2390–2399 (2022). https://doi.org/10.1134/S107036322211024X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107036322211024X