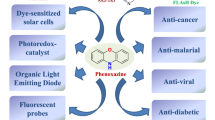
Abstract
Oxadiazoles are heterocyclic compounds in numerous approved, and experimental drugs, bioactive molecules, liquid crystals, diodes and in solar cell applications. It is one of the primary molecules studied in recent times and is a vital molecule employed in medicinal-chemistry for the preparation of anticancer, anti-HIV, anti-TB, anti-hepatitis etc. Research in the chemistry of the oxadiazole derivatives has been developed with the evolution of each application area and the need for target molecules with specific properties. A large number of in vivo applications for the oxadiazole analogues have also been performed. Presently, it has also been introduced as both auxiliary acceptor and spacer in dye-sensitized solar cell study with significant efficiency. Extensive literature has been documented the different applications of this versatile scaffold. The current review described some critical synthetic approaches to form such a vital moiety along with its derivatives and discussed its diverse applications, which includes the moiety in pharmacological activities, diode, liquid crystal, and solar cell fields. The review also describes some advancement in the field from our group, and also, the scope of the further study has been introduced.





Similar content being viewed by others
REFERENCES
Arora, P., Arora, V., Lamba, H., and Wadhwa, D., Int. J. Pharm. Sci. Res., 2012, vol. 3, pp. 2947–2955. https://doi.org/10.13040/IJPSR.0975-8232.3(9).2947-54
Czarnik AW., Acc. Chem. Res., 1996, vol. 29, pp. 112–113. https://doi.org/10.1021/ar950256n
Katritzky, A.R., Ley, S.V., Meth-Cohn, O., and Rees, C.W., Pergamon Publisher, 1995. ISBN-13: 978-0080406046.
Paraschivescu, C.C., Păun, A., and Matache, M., Targets Heterocycl. Syst., 2016, vol. 20, pp. 174–196. http://dx.medra.org/10.17374/targets.2017.20.174.
Wei, H., He, C., Zhang, J., and Shreeve, Jn.M., Angew. Chem., Int. Ed., 2015, vol. 54, pp. 9367–9371. https://doi.org/10.1002/anie.201503532
Sengupta, P., Mal, M., Mandal, S., Singh, J., and Maity, T.K., Iran. J. Pharmacol. Ther., 2008, vol. 7, pp. 165–160. http://ijpt.iums.ac.ir/article-1-173-en.html.
Bhardwaj, N., Saraf, S., Sharma, P., and Kumar, P., E.-J. Chem., 2009, vol. 6, pp. 1133–1138. ISSN: 0973-4945.
Zhou, Y., Wang, J., Gu, Z., et al. Chem. Rev., 2016, vol. 116, pp. 422–518. https://doi.org/10.1021/acs.chemrev.5b00392
Tan, T.M.C., Chen, Y., Kong, K.H., et al. Antiviral Res., 2006, vol. 71, pp. 7–14. https://doi.org/10.1016/j.antiviral.2006.02.007
Zuo W-Q., Wang N-Y., Zhu Y-X., et al. RSC Adv. 2016, vol. 6, pp. 40277–40286. https://doi.org/10.1039/c6ra01179a
Kemnitzer, W., Kuemmerle, J., Zhang, H.Z., et al., Bioorg. Med. Chem. Lett., 2009, vol. 19, pp. 4410–4415. https://doi.org/10.1016/j.bmcl.2009.05.052
Kumar, A., D’Souza, S.S., Gaonkar, S.L., Rai, K.M., and Salimath, B.P., Invest. New Drugs., 2008, vol. 26, pp. 425–435. https://doi.org/10.1007/s10637-008-9116-5
Kamal, A., Srikanth, Y., Shaik, TB., et al., MedChemComm., 2011, vol. 2, pp. 819–823. https://doi.org/10.1039/C0MD00177E
Formagio, A.S., Tonin, L.T., Foglio, M.A., et al. Bioorg Med Chem., 2008, vol. 16, pp. 9660–9667. https://doi.org/10.1016/j.bmc.2008.10.008
Kamal, A., Srikanth, P.S., Vishnuvardhan, M.V., et al., Bioorg. Chem., 2016, vol. 65, pp. 126–136. https://doi.org/10.1016/j.bioorg.2016.02.007
Islam, M., Siddiqui, A.A., Rajesh, R., Bakht, A., and Goyal, S., Acta Pol. Pharm., 2008, vol. 65, pp. 441–447. https://doi.org/10.12691/ajps-2-1-1
Kumar, D., Patel, G., Johnson, E.O., and Shah, K., Bioorg. Med. Chem. Lett., 2009, vol. 19, pp. 2739–2741. https://doi.org/10.1016/j.bmcl.2009.03.158
Ouyang, X., Piatnitski, E.L., Pattaropong, V., et al., Bioorg. Med. Chem. Lett., 2006, vol. 16, pp. 1191–1196. https://doi.org/10.1016/j.bmcl.2005.11.094
Chekler, E.L.P., Kiselyov, A.S., Ouyang, X., et al., ACS Med. Chem. Lett., 2010, vol. 1, pp. 488–492. .https://doi.org/10.1021/ml1001568
Jessen, K.A., English, N.M., Yu Wang, J., et al., Mol. Cancer Ther., 2005, vol. 4, pp. 761–771. .https://doi.org/10.1158/1535-7163.MCT-04-0333
Garg, S. and Raghav, N., Bioorg. Chem., 2016, vol. 67, pp. 64–74. https://doi.org/10.1016/j.bioorg.2016.05.003
Palmer, J.T., Hirschbein, B.L., Cheung, H., et al., Bioorg. Med. Chem. Lett., 2006, vol. 16, pp. 2909–2914. https://doi.org/10.1016/j.bmcl.2006.03.001
Khan, M.T., Choudhary, M.I., Khan, K.M., Rani, M., and Atta ur, R., Bioorg. Med. Chem., 2005, vol. 13, pp. 3385–3395. https://doi.org/10.1016/j.bmc.2005.03.012
Tillekeratne, L.M., Sherette, A., Fulmer, J.A., et al., Bioorg. Med. Chem. Lett., 2002, vol. 12, pp. 525–528. https://doi.org/10.1016/S0960-894X(01)00827-7
Lukas, T.J., Schiltz, G.E., Arrat, H., Scheidt, K., and Siddique, T., Bioorg. Med. Chem. Lett., 2014, vol. 24, pp. 1532–1537. https://doi.org/10.1016/j.bmcl.2014.01.078
Bhatt, P., Sen, A., and Jha, A., Chem. Select., 2020, vol. 5, pp. 3347–3354. https://doi.org/10.1002/slct.201904412
Bansal, S., Bala, M., Suthar, S.K., et al., Eur. J. Med. Chem., 2014, vol. 80, pp. 167–174. https://doi.org/10.1016/j.ejmech.2014.04.045
Kadi, A.A., El-Brollosy, N.R., Al-Deeb, O.A., Habib, E.E., Ibrahim, T.M., and El-Emam, A.A., Eur. J. Med. Chem., 2007, vol. 42, pp. 235–242. https://doi.org/10.1016/j.ejmech.2006.10.003
Albratty, M., El-Sharkawy, K.A., and Alhazmi, H.A., Acta Pharm., 2019, vol. 69, pp. 261–276. https://doi.org/10.2478/acph-2019-001508
Chawla, G., Naaz, B., and Siddiqui, A.A., Mini Rev. Med. Chem., 2018, vol. 18, pp. 216–233. https://doi.org/10.2174/1389557517666170127121215
Ewies, E.F., El-Hussieny, M., El-Sayed, N.F., Fouad, M.A., Eur. J. Med. Chem., 2019, vol. 180, pp. 310–320. https://doi.org/10.1016/j.ejmech.2019.07.029
Farshori, N.N., Rauf, A, Siddiqui, M.A., Al-Sheddi, E.S., and Al-Oqail, M.M., Arab. J. Chem., 2017, vol. 10, pp. S2853–S2861. https://doi.org/10.1016/j.arabjc.2013.11.010
Tantray, M.A., Khan, I., Hamid, H., Alam, M.S., Dhulap, A., and Kalam, A., Bioorg. Chem., 2018, vol. 77, pp. 393–401. https://doi.org/10.1016/j.bioorg.2018.01.040
Verma, G., Khan, M.F., Akhtar, W., Alam, M.M., Akhter, M., and Shaquiquzzaman, M., Mini Rev. Med. Chem., 2019, vol. 19, pp. 477–509. https://doi.org/10.2174/1389557518666181015152433
Glomb, T., Szymankiewicz, K., and Świątek, P., Molecules., 2018, vol. 23, pp. 3361-1–3361-16. https://doi.org/10.3390/molecules23123361
Sahu, V.K., Singh, A.K., and Yadav, D., Int. J. ChemTech Res., 2011, vol. 3, pp. 1362–1372. ISSN: 0974-4290.
Patel, K.D., Prajapati, S.M., Panchal, S.N., and Patel, H.D., Synth. Commun., 2014, vol. 44, pp. 1859–1875. https://doi.org/10.1080/00397911.2013.879901
Kumar, B., Kumar, A., Beheraand, A.K., and Raj, V., J. Cell Sci. Ther., 2016, vol. 7, pp. 233-1–233-7. https://doi.org/10.4172/2157-7013.1000233
Rubina, B., Dharam, P.P., Garima, K., Ravi, K., and Manni, D., Indian J. Pharm. Educ. Res., 2019, vol. 53, pp. S1–S16. https://doi.org/10.5530/ijper.53.2s.44
Bondock, S., Adel, S., Etman, H.A., and Badria, F.A., Eur. J. Med. Chem., 2012, vol. 48, pp. 192–199. https://doi.org/10.1007/s11164-015-2121-3
Desai, N., Dodiya, A.M., Rajpara, K.M., and Rupala, Y.M., J. Saudi Chem. Soc., 2014, vol. 18, pp. 255–261. https://doi.org/10.1016/j.jscs.2011.11.021
Li, Y., Luo, Y., Hu, Y., et al., Bioorg. Med. Chem., 2012, vol. 20, pp. 4316–4322. https://doi.org/10.1016/j.bmc.2012.05.050
Navarrete-Vazquez, G., Marı, G., Duarte-Fajardo, Z.V., et al., Bioorg. Med. Chem., 2007, vol. 15, pp. 5502–5508. https://doi.org/10.1016/j.bmc.2007.05.053
Parikh, K. and Joshi, D., J. Chem. Sci., 2014, vol. 126, pp. 827–835. https://doi.org/10.1007/s12039-014-0625-9
Raval, J.P., Akhaja, T.N., Jaspara, D.M., Myangar, K.N., and Patel, N.H., J. Saudi Chem. Soc., 2014, vol. 18, pp. 101–106. https://doi.org/10.1016/j.jscs.2011.05.019
Zhang F., Wang X.-L., Shi J., et al. Bioorg. Med. Chem., 2014, vol. 22, pp. 468–477. https://doi.org/10.1016/j.bmc.2013.11.004
De Oliveira, C.S., Lira, B.F., Barbosa-Filho, J.M., Lorenzo, J.G.F., Athayde-Filho, D., and Filgueiras, P., Molecules, 2012, vol. 17, pp. 10192–10231. https://doi.org/10.3390/molecules170910192
Dawle, J., Mathapati, S., Bondge, A., and Momin, K., Asian J. Res. Chem., 2012, vol. 5, pp. 707–711. https://doi.org/10.5958/0974-4150
Kudelko, A., Wróblowska, M., Jarosz, T., Łaba, K., Łapkowski, M., and Ann Arbor, M.I., Michigan Publishing, University of Michigan Library, 2015.
Nowrouzi, N., Khalili, D., and Irajzadeh, M., J. Iran. Chem. Soc., 2015, vol. 12, pp. 801–806. https://doi.org/10.1007/s13738-014-0542-3
Jasiak, K., Kudelko, A., Zieliński, W., and Kuźnikb, N., Arkivoc, 2017, vol. ii, pp. 87–106. https://doi.org/10.3998/ark.5550190.p009.668
Sirgamalla, R., Kommakula, A., Banoth, S., et al., Synth. Commun., 2018, vol. 48, pp. 954–962. https://doi.org/10.1080/00397911.2018.1432761
Shruthi, T., Eswaran, S., Shivarudraiah, P., Narayanan, S., and Subramanian, S., Bioorg. Med. Chem. Lett., 2019, vol. 29, pp. 97–102. https://doi.org/10.1016/j.bmcl.2018.11.002
Akram, M., Rauf, A., Saeed, A., et al., Trop. J. Pharm. Res., 2018, vol. 17, pp. 127–134. https://doi.org/10.4314/tjpr.v17i1.18
Upare, A.A., Gadekar, P.K., Sivaramakrishnan, H., et al., Bioorg. Chem., 2019, vol. 86, pp. 507–512. https://doi.org/10.1016/j.bioorg.2019.01.054
Lakshmithendral, K., Saravanan, K., Elancheran, R., et al., Eur. J. Med. Chem., 2019, vol. 168, pp. 1–10. https://doi.org/10.1016/j.ejmech.2019.02.033
Baykov, S., Tarasenko, M., Zelenkov, L.E., Kasatkina, S., Savko, P., and Shetnev, A., Eur. J. Org. Chem., 2019, vol. 2019, pp. 5685–5693. https://doi.org/10.1002/ejoc.201900843
Elancheran, R., Kabilan, S., Kotoky, J., Ramanathan, M., and Bhattacharjee, A., Combin. Chem. High Throughput Screen., 2019, vol. 22, pp. 307–316. https://doi.org/10.2174/1386207322666190701124
Cascioferro, S., Attanzio, A., Di Sarno, V., Musella, S., Tesoriere, L., Cirrincione, G., Diana, P., and Parrino, B., Mar. Drugs, 2019, vol. 17, pp. 35-1–35-15. https://doi.org/10.3390/md17010035
Benassi, A., Doria, F., and Pirota, V., Int. J. Mol. Sci., 2020, vol. 21, pp. 8692-1–8692-28. https://doi.org/10.3390/ijms21228692
Biernacki, K., Daśko, M., Ciupak, O., Kubiński, K., Rachon, J., and Demkowicz, S., Pharmaceuticals, 2020, vol. 13, pp. 111 (1–45). https://doi.org/10.3390/ph13060111
Polothia, R., Raolji, G.S.B., Kuchibhotla, V.S., Sheelama, K., Tuniki, B., and Thodupunuri, P., Syn. Comm. Rev., 2019, vol. 49, pp. 1603–1612.
Esser, C., Lawrence, B., Sherr, D., et al., Int. J. Mol. Sci., 2018, vol. 19, pp. 3603-1–3603-8. https://doi.org/10.3390/ijms19113603
Pitasse-Santos, P., Sueth-Santiago, V., and Lima, M.E., J. Braz. Chem. Soc., 2018, vol. 29, pp. 435–456. https://doi.org/10.21577/0103-5053.20170208
Tamoto, N., Adachi, C., and Nagai, K., Chem. Mater., 1997, vol. 9, pp. 1077–1085. https://doi.org/10.1021/cm960391+
Wu, C.-L., Chang, C.-H., Chang, Y.-T., Chen, C.-T., Chen, C.-T., and Su, C.-J., J. Mater. Chem. C, 2014, vol. 2, pp. 7188–7200. https://doi.org/10.1039/c4tc00876f
Shih, C.-H., Rajamalli, P., Wu, C.-A., Hsieh, W.-T., and Cheng, C.-H., ACS Appl. Mater. Interfaces., 2015, vol. 7, pp. 10466–10474. https://doi.org/10.1021/acsami.5b01872
Shih, C.-H., Rajamalli, P., Wu, C.-A., Chiu, M.-J., Chu, L.-K., and Cheng, C.-H., J. Mater. Chem. C, 2015, vol. 3, pp. 1491–1496. https://doi.org/10.1039/C4TC02348J
Fan, C., Lei, Y., Liu, Z., et al., ACS Appl. Mater. Interfaces, 2015, vol. 7, pp. 20769–20778. https://doi.org/10.1021/acsami.5b05815
Zhang, F., Li, W., Yu, Y., et al., J. Mater. Chem. C, 2016, vol. 4, pp. 5469–5475.
Zhang, J., Zhou, L., Al-Attar, H.A., et al., Adv. Funct. Mater., 2013, vol. 23, pp. 4667–4677. https://doi.org/10.1002/adfm.201300344
Lehmann, M., Köhn, C., Kresse, H., and Vakhovskaya, Z., Chem. Commun., 2008, pp. 1768–1770. https://doi.org/10.1039/B718348H
Qu, S., Chen, X., Shao, X., et al., J. Mater. Chem., 2008, vol. 18, pp. 3954–3964. https://doi.org/10.1039/B804189J
Wang, Y., Shi, J., Chen, J., Zhu, W., and Baranoff, E., J. Mater. Chem. C. 2015, vol. 3, pp. 7993–8005. https://doi.org/10.1039/c5tc01565k
Zheng, C., Yuan, A., Zhang, Z., Shen, H., Bai, S., and Wang, H., J. Fluoresc., 2013, vol. 23, pp. 785–791. https://doi.org/10.1007/s10895-013-1213-y
Liu, Q., Bian, W., Shi, H., et al., Org. Biomol. Chem., 2013, vol. 11, pp. 503–508. https://doi.org/10.1039/C2OB26888D
Li, A.F., Ruan, Y.B., Jiang, Q.Q., He, W.B., and Jiang, Y.B., Chem. Eur. J., 2010, vol. 16, pp. 5794–5802. https://doi.org/10.1002/chem.200903265
Paraschivescu, C.C., Hădade, N.D., Coman, A.G., Gautier, A., Cisnetti, F., and Matache, M., Tetrahedron Lett., 2015, vol. 56, pp. 3961–3964.
Kim, T.H., Lee, C.-H., Kwak, C.G., Choi, M.S., Park, W.H., and Lee, T.S., Mol. Cryst. Liq. Cryst., 2007, vol. 463, pp. 255/[537]–261/[543]. https://doi.org/10.1080/15421400601028054
Tang, L., Zheng, Z., Zhong, K., and Bian, Y., Tetrahedron Lett., 2016, vol. 57, pp. 1361–1364. https://doi.org/10.1016/j.tetlet.2015.05.005
Carli, S., Baena, J.P.C., Marianetti, G., et al., ChemSusChem., 2016, vol. 9, pp. 657–661. https://doi.org/10.1002/cssc.201501665
Mane, S.B., Cheng, C.-F., Sutanto, A.A., Datta, A., Dutta, A., and Hung, C.-H., Tetrahedron, 2015, vol. 71, pp. 7977–7984. https://doi.org/10.1016/j.tet.2015.08.068
Srinivas, K., Sivakumar, G., Kumar, C.R., et al., Synth. Met., 2011, vol. 161, pp. 1671–1681. https://doi.org/10.1016/j.synthmet.2011.06.001
Wang, Z., Zhang, H., Killian, B.J., et al., Eur. J. Org. Chem., 2015, vol. 2015, pp. 5183–5188. https://doi.org/10.1002/ejoc.201500583
Dacons, J.C. and Sitzmann, M.E., J. Heterocycl. Chem., 1977, vol. 14, pp. 1151–1155. https://doi.org/10.1002/jhet.5570140706
Klapötke, T.M. and Witkowski, TG., ChemPlusChem., 2016, vol. 81, pp. 357–360. https://doi.org/10.1002/cplu.201600078
Rashid, M., Husain, A., and Mishra, R., Eur. J. Med. Chem., 2012, vol. 54, pp. 855–866. https://doi.org/10.1016/j.ejmech.2012.04.027
Chrysina, E.D., Kosmopoulou, M.N., Tiraidis, C., et al., Protein Sci., 2005, vol. 14, pp. 873–888. https://doi.org/10.1110/ps.041216105
El-Emam, A.A., Al-Deeb, O.A., Al-Omar, M., and Lehmann, J., Bioorg. Med. Chem., 2004, vol. 12, pp. 5107–5113. https://doi.org/10.1016/j.bmc.2004.07.033
Almasirad, A., Tabatabai, S.A., Faizi, M., et al., Bioorg. Med. Chem. Lett., 2004, vol. 14, pp. 6057–6059. https://doi.org/10.1016/j.bmcl.2004.09.072
Kucukguzel, S.G., Oruc, E.E., Rollas, S., Sahin, F., and Ozbek, A., Eur. J. Med. Chem., 2002, vol. 37, pp. 197–206. https://doi.org/10.1016/S0223-5234(01)01326-5
Glomb, T., Szymankiewicz, K., and Świątek, P., Molecules, 2018, vol. 23, pp. 3361 (1–16). https://doi.org/10.3390/molecules23123361
Boström, J., Hogner, A., Llinàs, A., Wellner, E., and Plowright, A.T., J. Med. Chem., 2012, vol. 55, pp. 1817–1830. https://doi.org/10.1021/jm2013248
Verma, G., Chashoo, G., Ali, A., et al., Bioorg. Chem., 2018, vol. 77, pp. 106–124. https://doi.org/10.1016/j.bioorg.2018.01.007
Narella, S.G., Shaik, M.G., Mohammed, A., Alvala, M., Angeli, A., and Supuran, C.T., Bioorg. Chem., 2019, vol. 87, pp. 765–772. https://doi.org/10.1016/j.bioorg.2019.04.004
Boraei, A.T.A., Ashour, H.K., El Tamany, E.S.H., Abdelmoaty, N., El-Falouji, A.I., and Gomaa, M.S., Bioorg. Chem., 2019, vol. 85, pp. 293–307. https://doi.org/10.1016/j.bioorg.2018.12.039
Fathi, M.A.A., Abd El-Hafeez, A.A., Abdelhamid, D., Abbas, S.H., Montano, M.M., and Abdel-Aziz, M., Bioorg. Chem., 2019, vol. 84, pp. 150–163. https://doi.org/10.1016/j.bioorg.2018.11.032
Ananth, A.H., Manikandan, N., Rajan, RK., et al., Chem. Biodiversity, 2020, vol. 17, e1900659. https://doi.org/10.1002/cbdv.201900659
Lakshmithendral, K., Saravanan, K., Elancheran, R., et al., Eur. J. Med. Chem., 2019, vol. 168, pp. 1–10. https://doi.org/10.1016/j.ejmech.2019.02.033
Zhang, S., Luo, Y., He, L.Q., et al., Bioorg. Med. Chem., 2013, vol. 21, pp. 3723–3729. https://doi.org/10.1016/j.bmc.2013.04.043
Abd El-Meguid, E.A., Awad, H.M., and Anwar, M.M., Russ. J. Gen. Chem., 2019, vol. 89, pp. 348–356. https://doi.org/10.1134/S1070363219020282
Abdel-Aziz, M., Metwally, K.A., Gamal-Eldeen, A.M., and Aly, O.M., Anticancer Agents Med. Chem., 2015, vol. 16, pp. 269–277. https://doi.org/10.2174/1871520615666150907093855
Chi, K.Q., Wei, Z.Y., Wang, K.S., et al., Bioorg. Chem., 2017, vol. 75, pp. 157–169. https://doi.org/10.1016/j.bioorg.2017.09.013
Kumar, A., D’Souza, S.S., Nagaraj, S.R., Gaonkar, S.L., Salimath, B.P., and Rai, K.M., Cancer Chemother. Pharmacol., 2009, vol. 64, pp. 1221–1233. https://doi.org/10.1007/s00280-009-0992-y
Rajak, H., Agarawal, A., Parmar, P., et al., Bioorg. Med. Chem. Lett., 2011, vol. 21, pp. 5735–5738. https://doi.org/10.1016/j.bmcl.2011.08.022
Moniot, S., Forgione, M., Lucidi, A., et al., Med. Chem., 2017, vol. 60, pp. 2344–2360. https://doi.org/10.1021/acs.jmedchem.6b01609
Khanam, R., Ahmad, K., Hejazi, I.I., et al., Cancer Chemother. Pharmacol., 2017, vol. 80, pp. 1027–1042. https://doi.org/10.1007/s00280-017-3414-6
Altıntop, M.D., Sever, B., Akalın Çiftçi, G., Turan-Zitouni, G., Kaplancıklı, Z.A., and Özdemir, A., Molecules, 2018, vol. 23, pp. 1–6. https://doi.org/10.3390/molecules23061318
Zhang, Z.M., Zhang, X.W., Zhao, Z.Z., et al., Bioorg. Med. Chem., 2012, vol. 20, pp. 3359–3367.
Norouzi, S., Norouzi, M., Amini, M., et al., Asian J. Chem., 2009, vol. 21, pp. 7403.
Balaji, K., Bhatt, P., and Jha, A., Drug Res. (Stuttg.), 2016, vol. 66, pp. 587–591. https://doi.org/10.1055/s-0042-112028
Wang, J., Liao, L., and Li, Y., Nano Lett., 2018, vol. 18, pp. 7060–7065. https://doi.org/10.1021/acs.nanolett.8b03065
Bhatt, P., Kumar, M., and Jha, A., Mol. Diversity, 2018, vol. 22, pp. 827–840. https://doi.org/10.1007/s11030-018-9832-5
Khanam, R., Hejazi, I.I., Shahabuddin, S., Bhat, A.R., and Athar, F., J. Pharm. Anal., 2019, vol. 9, pp. 133–141. https://doi.org/10.1016/j.jpha.2018.12.002
ACKNOWLEDGMENTS
The authors are thankful to the GITAM (Deemed to be University) for providing instrumental and computational facilities.
Funding
We thank the Department of Science and Technology, India, under DST-FIST Program 2014 with project no. SR/FST/ETI-373/2016.
Author information
Authors and Affiliations
Contributions
Anjali Jha and Anik Sen contributed equally to the study.
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain studies that use humans and animals as objects of research.
Conflict of Interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Anjali Jha, Sen, A. & Malla, R.R. Chemistry of Oxadiazole Analogues: Current Status and Applications. Russ J Bioorg Chem 47, 670–680 (2021). https://doi.org/10.1134/S1068162021030092
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162021030092