Abstract
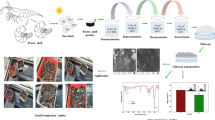
The present study deals with the penetration pathways of radiolabeled chitosan and its nanoparticle complexes in refrigerated huso fillets and their relationship with main physical and chemical parameters of the fillet after exposing the fillets for 7 and 12 days to 0.5 and 1% solutions of chitosan (Ch) and its nanoparticles (ChNPs) using the 67Ga labeling technique. Radiolabeling of chitosan and its nanoparticles was performed using 67GaCl3 as the starting radionuclide form. Radiolabeled Ch and ChNPs complexes were experimentally evaluated in refrigerated huso fillets, and 1% ChNPs showed the best penetration depth. The best outcomes for the quality traits were also obtained with 1% ChNPs. ChNPs can be used to preserve the quality of the huso fillet.



Similar content being viewed by others
REFERENCES
Hussain, M., New Zealand Food Technol., 2013, vol. 48, p. 33. https://doi.org/10.3390/foods2040585
Roman, S., Sanchez-Siles, L.M., and Siegrist, M., Trends Food Sci. Technol., 2017, vol. 67, pp. 44–57. https://doi.org/10.1016/j.tifs.2017.06.010
Amit, S.K., Uddin, M.M., and Rahman, R., Agricult. Food Secur., 2017, vol. 6, p. 51. https://doi.org/10.1186/s40066-017-0130-8
Dehghani, S., Hosseini, S.V., and Regenstein, J.M., Food Chem., 2018, vol. 240, pp. 505–513. https://doi.org/10.1016/j.foodchem.2017.07.034
Kurita, K., Marine Biotechnol., 2006, vol. 89, pp. 2203–2226. https://doi.org/10.1007/s10126-005-0097-5
Britto, D., and Assis, O.B.G., Int. J. Biol. Macromol., 2007, vol. 41, pp. 198–203. https://doi.org/10.1016/j.ijbiomac.2007.02.005
Tian, M., Ticer, T., and Wang, Q., Small, 2020, vol. 16, no. 10, ID 1904064. https://doi.org/10.1002/smll.201904064
Du, W.L., Niu, S.S., and Xu, Y.L., Carbohydr. Polym., 2009, vol. 75, pp. 385–389. https://doi.org/10.1016/j.carbpol.2008.07.039
Kumar, S., Ye, F., and Dobretsov, S., Appl. Sci., 2019, vol. 9, ID 2409. https://doi.org/10.3390/app9122409
Zhang, Y., Yang, Y., Tang, K., J. Appl. Polym. Sci., 2008, vol. 107, pp. 891–897. https://doi.org/10.1002/app.26402
Ramezani, Z., Zarei, M., and Raminnejad, N., Food Control, 2015, vol. 51, pp. 43-48. https://doi.org/10.1016/j.foodcont.2014.11.015
Hosseini, S.V., Abedian‐Kenari, A., Rezaei, M., Nazari, R.M., Feás, X., and Rabbani, M., Food Chem., 2010, vol. 118, no. 2, pp. 341–348. https://doi.org/10.1016/j.foodchem.2009.04.131
Kitson, S.L., Moody, T., and Watters, W., Defacto Healthy Reputations. http://www.outsourcedpharma.com/doc/modern-developments-in-isotopic-labelling-0001.
Jalilian, A.R., Yousefnia, H., Shafaii, K., Novinrouz, A., and Rajamand, A.A., Iran. J. Pharm. Res., 2012, vol. 11, no. 2, pp. 523–531. https://doi.org/10.22037/IJPR.2012.1090
Alishahi, A., Mirvaghefi, A., Tehrani, M.R., Farahmand, H., Shojaosadati, S.A., Dorkoosh, F.A., and Elsabee, M.Z., Food Chem., 2011, vol. 126, pp. 935–940. https://doi.org/10.1016/j.foodchem.2010.11.086
Alishahi, A., Mirvaghefi, A., Tehrani, M.R., Farahmand, H., Koshio, S., Dorkoosh, F.A., and Elsabeed, M.Z., Carbohydr. Polym., 2011, vol. 86, pp. 142–146. https://doi.org/10.1016/j.carbpol.2011.04.028
Akhlaghi, M., and Pourjavadi, A., Nukleonika, 2011, vol. 56, no. 1, pp. 41−47.
Goulas, A.E., and Kontominas, M.G., Food Chem., 2005, vol. 93, pp. 511–520. https://doi.org/10.1016/j.foodchem.2004.09.040
Egan, H., Kirk, R., and Sawyer, R., 1997, pp. 609–634.
Suvanich, V., Jahncke, M.L., and Marshall, D.L., Food Science, 2000, vol. 65, no. 1, pp. 24–29. https://doi.org/10.1111/j.1365-2621.2000.Tb15950.x
Siripatrawan, U., and Noipha, S., Food Hydrocolloids, 2012, vol. 27, pp. 102–108. https://doi.org/10.1016/j.foodhyd.2011.08.011
Ojagh, S.M., Rezaei, M., Razavi, S.H., and Hosseini, S.M.H., Food Chem., 2010, vol. 120, pp. 193–198. https://doi.org/10.1016/j.foodchem.2009.10.006
Eymard, S., Baron, C.P., and Jacobsen, C., Food Chem., 2009, vol. 114, pp. 57–65. https://doi.org/10.1016/j.foodchem.2008.09.030
Fan W., Sun J., Chen Y., Qiu, J., Zhang, Y., and Chi, Y., Food Chem., 2009, vol. 115, no. 1, pp. 66–70. https://doi.org/10.1016/j.foodchem.2008.11.060
Sathivel, S., Liu, Q., Huang, J., and Prinyawiwatkul, W., J. Food Eng., 2007, vol. 83, pp. 366–373. https://doi.org/10.1016/j.jfoodeng.2007.03.009
Li, T., Hu, W., Li, J., and Zhang, X., Food Control, 2011, vol. 25, no. 1, pp. 101–106. https://doi.org/10.1016/j.foodcont.2011.10.029
Maghami, M., Motalebi, A.A., and Anvar, A.A., Food Sci. Nutrit., 2019, vol. 7, pp. 3030–3041. https://doi.org/10.1002/fsn3.1161
.Hong, H., Zhang, Y., Sun, J., and Cai, W., Nano Today, 2009, vol. 4, no. 5, pp. 399–413. https://doi.org/10.1016/j.nantod.2009.07.001
Bunis, D., Card, C., Lin, J., and Rhee, S., eCommons. Open Scholarship at Cornell, 2012. https://hdl.handle.net/1813/29684.
ACKNOWLEDGMENTS
This research was funded by the Gorgan University of Agricultural Sciences and Natural Resource and Nuclear Science and Technology Research Institute.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kamali, N.D., Alishahi, A.R., Heidarieh, M. et al. Evaluation of the Relationship between Penetration Percent of Chitosan and Its Nanoparticles and Qualitative Traits in Huso huso Fillet Using 67Ga Radiolabeling of Chitosan. Radiochemistry 64, 776–782 (2022). https://doi.org/10.1134/S1066362222060157
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1066362222060157